Private Actions and Personal Liability

Fig. 7.1
Common state law claims in foodborne illness litigation
Causation and Res Ipsa Loauitor
Causation provides a difficult hurdle for plaintiffs. Often plaintiffs will rely on FDA and CDC investigations into outbreaks, which typically will identify the responsible firm. Then experts are called to provide testimony aimed at attributing negligence of the firm as the cause of the outbreak. To prove negligence, plaintiffs must chow causation, which means the defendant’s negligence is more likely than not the cause of their injury (Polin 1998).1 How causation is demonstrated depends on State law. Typically a three prong approached is used under the theory of res ipsa loquitor ( see Fig. 7.3) . This doctrine looks at three points:
1.
The incident was caused by “an agency or instrumentality under the exclusive control” of the defendant.
2.
The incident must be the type that ordinarily does not happen unless someone is negligent.
3.
The incident cannot be “due to any voluntary act or contributory fault of the plaintiff.” ( Miller Meat Co.)2
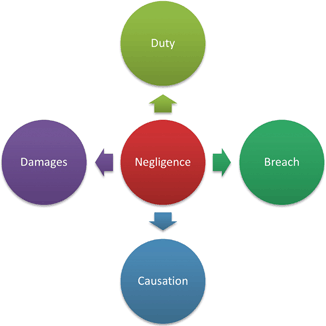
Fig. 7.2
Elements of a negligence claim
In Ford the court reviewed a negligence action against Miller Meat Company after the plaintiff broke a tooth on a bone fragment in a ground beef product produced by Miller Meat. The court using the three-prong test found that the plaintiff failed to show negligence. In particular the test noted the size of the bone fragment, which was around one-eighth of an inch, was so small that “no one could reasonably expect the vendor to remove [it] from ground beef” ( Miller Meat Co.).3 The decision in Ford is not only instructive on the elements of causation, but also signals to potential plaintiffs of how fact dependent negligence cases can be.
One concern with causation is whether the foodborne illness is attributable to the defendant’s products and not another source. In some cases bacteria responsible for foodborne illnesses can remain dormant for two or more weeks before causing any symptoms. This was the case when a plaintiff sued Burger King after allegedly becoming ill eating a Whopper (Hairston v. Burger King Corp.).4 The court dismissed the case largely on expert witness who testified that the plaintiff’s illness could have been caused by something eaten an hour to a week before feeling ill (Hairston v. Burger King Corp.)5. The court reasoned:
The trial court did not rule that Hairston did not have food poisoning, but found that she failed to carry her burden of proving that her condition was caused by consuming the Whopper. Kamberov testified that the cause of the food poisoning could have been anything Hairston ate from one hour to one week before she started having problems. Additionally, there was no evidence that any of the other 800 people who consumed a Whopper that day developed food poisoning. Hairston’s testimony merely established she was having severe gastric problems and that she had eaten a Whopper. Based on the entirety of the evidence, the trial court was not manifestly erroneous in determining that eating the Whopper did not cause Hairston’s gastric distress.
Issues also arise when plaintiff’s symptoms are chronic rather than acute. This was the case for a couple who sued a local restaurant, the Log Cabin a/k/a “Betty’s,” after becoming ill immediately following their dinner at the seafood buffet (Burnett v. Essex Insurance Company).6 In addition to their acute reaction to the food one plaintiff continued to complain of chronic abdominal pain (Burnett v. Essex Insurance Company).7 The court dismissed the case finding that their physician could not rule out other causes of the illness including treatment for similar symptoms prior to eating at Betty’s. The court held:
Dr. Ghanta testified that if the Plaintiffs’ history were correct, then he would relate their symptoms to the food consumed at the restaurant. However, Dr. Ghanta could not identify the source of the infection, and he could not rule out as other possible causes the local drinking water or from elsewhere in the community. Although a plaintiff need not scientifically identify the infection-producing organism to recover in a food poisoning case, he must still prove causation by a preponderance of the evidence. When the lack of more specific evidence of causation is considered in light of the Burnetts’ medical histories, we find no error in the trial court’s conclusion that they did not meet this burden. The evidence simply does not preponderate in their favor, given their propensity to gastric disorders and Dr. Ghanta’s reliance solely on their accounts to formulate his opinion of causation.
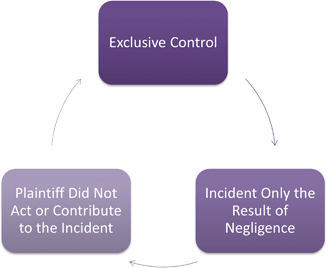
Fig. 7.3
Res Ipsa Loquitur elements
There are numerous disadvantages to foodborne illness litigation. This often results in only the most severe instance of illness or death or egregious acts being brought to court. In other words this type of case is better suited for large outbreaks where class action status may be available. Litigation can be expensive and take several years to reach a resolution. The compensation, typically from the insurance companies, largely covers legal fees and court costs. Given these restraints the individual suits for foodborne illness are not brought to court.
FSMA and Negligence
The Food Safety Modernization Act may slightly ease a plaintiff’s burden in negligence cases. The issue of causation will remain troublesome and difficult to prove. It may be aided by FSMA, which requires extensive record keeping that could prove to be a fertile ground for discovery. FSMA also requires new preventative measures and controls to be put in place. For example, the Preventative Controls rule discussed in Chapters 3 and 5 will require a food safety plan that outlines hazards and control of those hazards. This could be a roadmap for plaintiff’s attorneys searching for the elements of a negligence claim. Other rules pose similar risks. Smaller provisions of the rule may also be magnified in negligence claims. The use of high risk facility designation or the use of mandatory recall authority could aid in both inferring causation and proving failure to follow preventative controls. FSMA by no means requires perfection, but the level of documentation will be both, a rich source for finding the elements of a negligence claim and also a basis to infer the lack of documentation indicating improper hazard control and thus likely causation of the incident. All of this remains to be tested in the court.
7.2.2 Labeling Litigation
The Origins of Labeling Litigation
Labeling lawsuits are barely a decade old. Unlike previous chapters that explored early case law, ranging from 1911 through the 1930s, 1940s, 1950s and on, labeling litigation is new to the food law scene. Labeling litigation’s origins lie in 2005 following a precipitous drop in FDA enforcement of labeling and a corresponding increase in creative and non-compliant claims.
The market was ripe for the wave of new claims seen beginning in early 2000. The CDC began to issue reports on obesity in US adults, which the media began to report on and describe as an epidemic. The focus shifted to childhood obesity as an American epidemic. As the drumbeat of bad news carried on, a demand to surge for healthier foods began. Chapter 4 described the market share for “all natural” products, currently at US$ 41 billion, and “organic” currently at US$ 9 billion. The market demand is geared toward local, natural, and pure. It curiously demands that its processed foods should be as unprocessed as possible.
The FDA watched as the market flooded with new “eye-catching” claims but did little to enforce existing regulations. Amid this backdrop of market demand for more wholesome products, manufacturers began labeling products “all natural” “GMO free” “whole grains” or some other statement to imply the product was minimally processed or otherwise pure. The labeling created what one reporter described as a “halo effect” where the claims lead to a perception of healthfulness (NY Times 2008).8 Consumers would accept the claims and not make any additional assessment about the wholesome or healthfulness of the product. Trusting the claims consumers ate the foods often exceeding the serving size because of a mistaken belief that the product was healthy. The FDA noted in a statement, “…with consumers’ growing interest in eating healthy, we’ve seen the emergence of eye-catching claims and symbols on the front of food packages that may not provide the full picture on their products true nutritional value” (Hamburg 2010).9 The FDA, however, could not effectively enforce the market.
It is in this context that the first labeling lawsuit arose in 2005. In 2004 the non profit consumer advocacy group Center for Science in the Public Interest (CSPI) established a litigation department. The mission of the new litigation department would be “to fill the void left by the inactive government agencies” (CSPI Litigation). CSPI began pursuing a number of labeling claims and securing victories either in court or by settlement. It reached its first settlement in 2005 against Aunt Jemima’s corporate parent Pinnacle Foods. It sought a misbranding and quasi-economic-adulteration case against Pinnacle for its blueberry waffles made suing “blueberry bits.” In the settlement Pinnacle agreed to label the product as “artificially flavored” and stated t that he blueberries were imitations. The suits and settlements by CSPI and others would continue to grow.
The cases multiplied rapidly. One estimate placed the number of labeling lawsuits at only 19 federal court cases in 2008 (U.S. Chamber 2013).10 In the span of 4 years the number would grow to more than 102 federal court cases. The number continues to swell with many commentators now describing food labeling as replacing the era of litigation targeting tobacco companies with class actions.
The large number of cases can be difficult to sift through. It is helpful to separate the cases into three categories ( see Fig. 7.4). The first category involves cases making claims that are legal but unregulated. In this category are claims like “all natural”, “nutritious”, or “healthful”, which are permitted by the FDA under informal policy or no policy or regulation, but which are misleading. The second category centers on claims that violate State law equivalents to the FD&C and FDA regulations. Common claims for this category involve health and nutrient content claims, which are regulated by the FDA but also may violate State equivalent regulations or statutes. A third category involves food fraud. This unique category involves cases where a product claims to contain particular ingredients or amounts of ingredients, but upon analysis does not contain the ingredient, or not at the levels claimed.
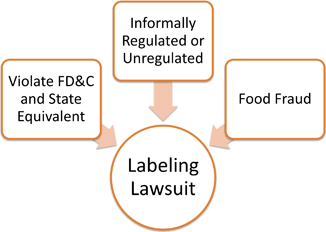
Fig. 7.4
Labeling litigation categories
“Natural” Suits
Natural lawsuits involve products labeled with claims like “all natural” on products containing some unnatural ingredient. This type of case fits in category one chiefly because the claim “all natural” is only subject to an informal policy. Products challenged using the “all natural” label typically contain artificial preservatives, high fructose corn syrup (HFCS), genetically modified organisms (GMOs), or other chemicals or unnatural ingredients ( see Fig. 7.5).
The FDA refuses to issue a regulation defining the term “natural” under NLEA. The Agency considered issuing a rule in 1993, but cited resource limitations and other priorities as it abandoned the effort (Fed. Reg. 1993).11 Instead, the FDA adopted an informal policy which would assess the use of the term “natural” on a case-by-case basis. There would be no formal rule adopted. This decision has left courts to interpret the meaning of natural under the Act, often when considering labeling challenges.
Dozens of cases involve claims with some variant on “all natural.” Given the frequent number of settlements there are only a few district or Circuit Court opinions to reference. Those Circuit Court options that are available frequently focus on the issue of preemption rather than a lengthy discussion of the substantive issue of whether the claims were misleading. Settlements for “all natural” claims reported in the media include most major brands. Kellogg’s Kashi brand, for example, settled after a class action challenged the use of “all natural” and “nothing artificial” on products containing a long list of substances like pyridoxine hydrocholoride and tocopherols (American Bar Association).12 Ben & Jerry’s is another well-known brand sued for its claim that its ice cream was “all natural” (Ben & Jerry’s Complaint).13 Kellogg’s also agreed to stop using the “all natural” claim. The complaint cited ingredients such as alkalized cocoa and other processed ingredients (Kashi Complaint).14 The company settled for US$ 5 million. The lawsuits benefited from the FDA informal policy.
A number of cases specifically focused on whether the use of HFCS qualified as “natural.” A case involving Snapple sought damages from the drink maker on the basis that it used HFCS and labeled its products “natural.” HFCS, as the argument holds are not like natural sugars, but are unnatural or processed. Snapple is rare in that the plaintiffs lost, not because the court deemed HFCS “natural,” but because they could not show that they suffered an economic harm by paying a premium for Snapple’s “natural” drinks. Snapple did drop the claim from its label shortly after the suit was dismissed. Not the desired pay-day sought by the plaintiff’s, but it could be described as effective private regulation.
Recent “natural” suits focus on the use of GMOs. Here again the FDA does not have a regulation or definition for GMOs. It regulates GMOs the same as any other food. The lawsuits, however, see a distinction calling GMOs unnatural foods. ConAgra Foods, offers an example of a GMO “natural” suit. ConAgra sells its Wesson line of cooking oils with the claim that it’s “100 % natural” (Food Safety News).15 The complaint asked the court to find the GMO ingredients in the product artificial or non natural, thus making the claim misleading. Other GMO suits involve General Mills and Frito Lay. There is yet to be a definitive answer on the subject from the courts.
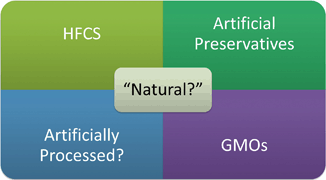
Fig. 7.5
Illustration of questions raised in “Natural” suits
Unregulated Misleading Claims
A number of labeling lawsuits allege false or misleading statements that are not regulated by the FDA such as “wholesome” or “balanced breakfast.” This type of suit also fits into category one. Critics of this type of lawsuit argue that the suits are frivolous with no true deception because the ingredients listing and nutrition fact panel provide the consumer all the necessary information to understand the context of the claim. Courts often reject this view finding compliance with ingredient listing and fact panels that cannot cure misleading claims without making the misbranding provisions of the Act superfluous. Unlike the “natural” claims cases where the question is whether the product truly is natural, the question in this type of case is whether the product actually is “wholesome” or “healthy.”
Nutella provides a much talked about example of this type lawsuit. Nutella maker, Ferrero USA Inc. settled for US$ 3 million following a class action law suit alleging that it mislead consumers with deceptive claims (NPR).16 The claims in question states that the Nutella spread was part of “balanced breakfast” and described the product as “healthy” and “nutritious.” Not mentioned in its labeling claims or marketing was its high sugar and fat content. This lead the lead plaintiff to decry the product that was the “next best thing to a candy bar” and far from the healthy, balanced snack depicted in marketing (NPR).17 Despite compliance with ingredient listing and nutrition facts Ferrero saw risk in defending the suit and settled.
Many other lawsuits follow a similar pattern as Nutella. Tropicana was sued for its claim on orange juice products that stated it was “100 % pure” and “natural” (Tropicana Complaint).18 Plaintiffs argued this was misleading because the juice was pasteurized, deaerated, colored, and flavored. Quaker Oats faced a lawsuit alleging that its claims on oatmeal and oatmeal bars were misleading (Quaker Oats Complaint).19 A wide variety of claims were cited by plaintiff’s including “wholesome” and “All the Nutrition of a Bowl of Instant Oatmeal.” The plaintiffs pointed to partially hydrogenated oils, a form of trans fats as an evidence that the claims mislead the public about the nutrition and healthfulness of its products. As can be seen, its not incredibly novel claims that are causing trouble. It is an odd combination of modern processed food ingredients with claims typically used for unprocessed products.
A surge of suits focused on the use of evaporated cane juice (ECJ), on labels instead of sugar or dried cane syrup. Plaintiffs brining suits challenging the use of ECJ stated it sounded like a healthier product, but they actually hide the sugar content. They also argued it violated the FDA’s standard of identity regulations. Suits were brought against Chobani and Trader Joe’s to name a few. Prior to the suits, the FDA issued a Draft Guidance, which is the lowest rung of authority the Agency can use, stating that the term juice does not encompass ECJ (FDA 2014).20 It never finalized the Guidance, but did issue warning letters stating that the use of ECJ was false and misleading because it did not adequately inform the consumer that the product was sugars or syrups and not juices.
Unsubstantiated Health and Nutrition Claims
In the second category of suits are two related types of lawsuits. The claims in question are health or nutrient content claims, but the wrongdoing varies between unsubstantiated and unauthorized. If the claim is an unauthorized claim it is one that failed to follow the procedure set by the FDA under NLEA. If it is an unsubstantiated claim it simply is not supported by current studies or science.
CSPI sued several major U.S. companies for unsubstantiated health and nutrition claims. In 2007, it sued Coca-Cola and Nestlé for claims made on a green tea drink called Enviga (CSPI Press Release).21 Enviga was labeled “the calorie burner” and in marketing materials states it aided in weight loss. The claims went so far as to claim the product contained “negative calories.” In its suit CSPI alleged the claims were unsupported and lacked any evidence that consumers would experience any calorie burning benefit. The suit led to a settlement and investigations by 28 States Attorneys General.
In another example from CSPI it threatened suit against Smart Balance, Inc. for labeling on its Smart Balance Blended Butter Sticks. The butter sticks were labeled prominently with the claim “Plant Sterols to Help Block Cholesterol in the Butter” (CSPI Press Release 2014).22 This type of claim dances on the line between health claim and disease or new drug claim. In characterizing the relationship between an ingredient and an effect on a disease it was also a health claim. CSPI alleged that the health claim did not follow the FDA regulations and was misleading because the products contained a disqualifying amount of saturated fat to be a health claim.
The proper use of health claims can provide a potential shield from labeling litigation. Yet, the misuse of such claims provides fodder for consumer advocacy groups and private plaintiffs to bring suit. Typically the fear of noncompliance would be a regulatory correction from the FDA. In the current environment, concern is secondary to potential exposure to litigation. Occasionally the FDA will follow-up on labeling litigation or settlements with warning letters. More commonly litigation quickly follows a Warning Letter.
Unauthorized Nutrient Content Claims
Another class of nutrient content claims rather that not following the requirements of a regulation are completely unauthorized. As discussed in Chapter 4 only the nutrient content claims provided in the regulations may be used on labeling. If a desired claim, which qualifies as a nutrient content claim, is not in the regulations then a process must be followed to gain approval. In some cases the unauthorized health claims border on disease claims. Typically, one would expect the FDA to enforce the use of unapproved nutrient content claims. The Agency does issue warning letters, but continues to struggle keeping pace with the market.
An example of a warning letter leading to a labeling lawsuit is seen in the case of Cheerios. The cereal from General Mills received a warning letter in 2009 for the use of unauthorized health claims. The cereal maker boldly stated, “you can Lower Your Cholesterol 4 % in 6 weeks.” The FDA not only found the claim to be an unauthorized health claim, but also demonstrating an intended use to “prevent, mitigate, or treat” hypercholesterolemia and coronary heart disease. A swarm of class action labeling lawsuits shortly followed the FDA publication of the letter (Brookings).23
A 2012 lawsuit against Bumble Bee provides another lesson stating the use of unauthorized health claims. In 2012 a lawsuit alleged Bumble Bee made unapproved nutrient content claims about the properties of Omega 3 (Bumble Bee Complaint).24 It labeled products as “Rich in Natural Omega-3” or “Excellent Source of Omega-3” which were not previously authorized by the FDA. The use of “rich” and “excellent” are similar to the use of “good source” under NELA. These types of claims need a daily value to compare what level is expected in the diet. The FDA has not established a daily value for Omega-3. There was no defense for Bumble Bee using the claim since it lacked authorization and could not point to persuasive authority as there was no daily value recommended for the ingredient.




Food Fraud
Food fraud can take many forms. The term fraud carries a negative connotation that may suggest an intentional deception or other nefarious act. In most cases food fraud involves economic adulteration. The pure push to reduce costs and increase profits leads to a conflicting aim to claim wholeness while using the cheapest ingredients possible. Other claims, likewise seeking to gain market share, stretch product’s attributes to the limit. When these claims fall flat consumers sue for fraud. In most cases the issue is not safety, but solely deception. For example, products falsely claiming to contain superior or healthy ingredients, or offering benefits not seen by a consumer or supported by any scientific analysis.
There are numerous examples of food fraud suits alleging some form of economic adulteration. The introduction to this topic provided the example of Aunt Jemima’s blue berry waffles only containing “blue berry bits.” The suit against an importer of grape seed oil sought damages because the product contained less than 25 % grape seed oil (Marquez Complaint).25 A similar lawsuit against Kangadis Food Inc. alleges it passed off pomace oil as “100 % Pure Olive Oil.” This type of case is rooted solely in State law (Class Action Notice).26 There is typically no need to cite FDA regulations or provisions of the Act. The case simply asks whether consumers were defrauded by the products.
One of the largest settlements involved food fraud. In 2010 Dannon settled for US$ 45 million when confronted with a suit alleging false and misleading claims for its Activia probiotic yogurt (ABC News).27 The Activia and DanActive brands made claims that their yogurt was “clinically” and “scientifically” proven to improve digestion and boost immunity. Dannon was unable to prove that the claims were anything other than empty marketing ploys.
Preemption
One tool to defend against a labeling lawsuit is preemption. It is perhaps the only instrument to shield a manufacturer from a full trial or more likely a settlement in lieu of trial. Preemption, as described in Chapter 4, is a doctrine unique to the U.S. It holds private actions, which rely on State law, which may not proceed if preempted by federal law. A handful of labeling lawsuits raised the challenge to stave off a settlement or trial.
Preemption is a complex area of law involving several grounds to bar State causes of actions. Preemption can be expressed, where Congress in the federal statute states an intent to preempt State law. State causes of action may not be barred if the parallel claim exception applies. The parallel claim exception allows a State law claim that is identical, or parallel, to the requirements of the federal law. Implied preemption evaluates the Congressional intent of passing legislation to determine if preemption was implied. Field preemption occurs where Federal regulation is so pervasive as to dominate the field leaving no room for State law, parallel or otherwise. Finally, conflict preemption describes the scenario where Federal and State law conflict make compliance between an either–or, but not both. This type of preemption can also be expressed or implied. ( see Fig. 7.6)
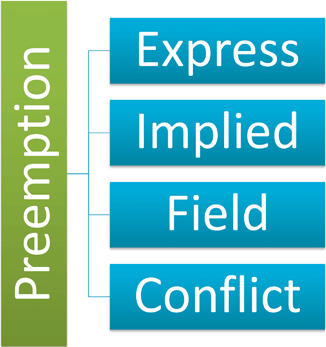
Fig. 7.6
Types of preemption
Conflict preemption provides insight into the FDA’s role in “all natural” lawsuits. The Snapple example provided above was challenged in court ( Snapple).28 Snapple argued that the claims were preempted since it could not comply with both, State law and the FDA’s informal policy and enforcement history. The court found that the FDA’s informal policy was insufficient, lacking “preemptive weight.” ( Snapple).29 The court noted the FDA did not appear settled and the definition could change. This varies from the express preemption in NELA which remains settled since its enactment. Snapple waived its right to express preemption, but did attempt to state that HFCS was an artificial color and preempted the State law claim. The court did not decide the issue but noted that the FDA treats HFCS as a sweetener not a flavoring ( Snapple).30 In the absence of clear definitive policy from the FDA, defining the term “all natural” preemption will be a defense subject to the case precedent of the district and circuit courts. This provides little predictability for firms making such claims or facing these suits.
Holk v. Snapple Beverage Corp., 575 F.3d 329
SMITH, Circuit Judge.
This appeal presents three issues related to the federal preemption of state causes of action. Plaintiff-appellant Stacy Holk brought several state law claims against defendant-appellee the Snapple Beverage Corporation in the Superior Court of New Jersey. After removing Holk’s lawsuit to the United States District Court for the District of New Jersey, Snapple sought to dismiss Holk’s complaint on, inter alia, the grounds of express preemption, implied field preemption, and implied conflict preemption. The District Court granted Snapple’s motion on the basis of implied preemption. For the reasons discussed below, we will reverse.
I.
…
B.
Snapple Beverage Corporation (“Snapple”) manufactures a variety of beverages, including a number of juice and tea-based drinks. In its marketing and advertising materials, Snapple represents that these beverages are “All Natural.” As the FDA has acknowledged, “[t]he word `natural’ is often used to convey that a food is composed only of substances that are not manmade and is, therefore, somehow more wholesome.” Food Labeling: Nutrient Content Claims, General Principles, Petitions, Definition of Terms, 56 Fed.Reg. 60,421, 60,466 (Nov. 27, 1991). Snapple products, however, contained high fructose corn syrup (“HFCS”), an ingredient manufactured from processed cornstarch.
Stacy Holk bought two bottles of Snapple on May 4, 2007. She paid $ 1.09 for each bottle. She had purchased other Snapple products over the preceding 6 years. Holk contends that the labels on these products are deceptive. She argues that consumers “have been, and continue to be, easy prey for Snapple’s unlawful activities because of their willingness to pay a premium price for foods and beverages, including Snapple beverages, that are represented to be ‘All Natural.’”
C.
Holk filed a class action lawsuit against Snapple in the Superior Court of New Jersey, asserting claims on the basis of: (I) the New Jersey Consumer Fraud Act; (II) unjust enrichment and common law restitution; (III) breach of express warranty; and (IV) breach of the implied warranty of merchantability. Holk’s claims were predicated on her belief that a number of statements on Snapple’s labels were misleading. She argued that (1) Snapple products were not “All Natural” because they contained HFCS; (2) Snapple products were not “Made from the Best Stuff on Earth,” as indicated on the label; and (3) Snapple falsely labeled some beverages, for example, calling one drink “Acai 333*333 Blackberry Juice,” despite the fact that the drink contained neither acai berry juice nor blackberry juice.
Snapple removed the case to the United States District Court for the District of New Jersey pursuant to the Class Action Fairness Act, 28 U.S.C. 1453(b). It then filed a motion to dismiss. The parties subsequently agreed that Holk could amend her complaint, rather than respond to Snapple’s motion. In October 2007, Holk filed an Amended Complaint, which reasserted that Snapple’s labels were misleading because they claimed the products were “All Natural” and because Snapple advertised some products as containing juice that was not in the beverages. The Amended Complaint did not allege any claims based on Snapple’s use of the phrase “Made From the Best Stuff on Earth.” Snapple filed a second motion to dismiss, arguing that Holk’s claims were preempted, that the claims should be dismissed under the doctrine of primary jurisdiction, and that the allegations failed to state a claim. Holk responded by dropping the argument related to the juice components of Snapple beverages, leaving only the claim that Snapple products containing HFCS were deceptively labeled “All Natural.”
The District Court heard oral argument on Snapple’s motion to dismiss in June 2008. On June 12, 2008, the District Court dismissed Holk’s complaint. It held that Snapple’s claims were preempted. In its opinion, the District Court correctly identified and discussed the three types of preemption. It also noted that Snapple argued that all three types of preemption were present in this case, as Snapple contended that (1) NLEA expressly preempted state labeling requirements that are not identical to federal requirements; (2) the comprehensive nature of the FDCA and its implementing regulations demonstrate that Congress intended the federal government to occupy the field; and (3) that state law stands as an obstacle to the purposes underlying the FDCA. Next, the District Court rejected Snapple’s express preemption argument, stating that there was not “specific preemptive language” in the FDCA that covered the claims. Nonetheless, the Court ruled that “Plaintiff’s claims in this case are impliedly preempted by the detailed and extensive regulatory scheme established by the [FDCA] and the FDA’s implementing regulations.”
The District Court stated that the FDA has used the broad authority granted to it under the FDCA to issue comprehensive regulations governing the labeling and naming of juice drinks. The Court declared that the comprehensive nature of these regulations demonstrate that “the FDA has carefully balanced beverage industry and consumer interests and created a complex regulatory framework to govern beverage labeling.” Though it acknowledged that the FDA has not defined “natural,” it found that the “FDA has in fact contemplated the appropriate use of the term,” as indicated by the FDA’s definition of “natural flavor” and its informal policy regarding use of the term “natural.” The Court also noted that the FDA has the authority to enforce the FDCA and regulations issued pursuant to it. In the Court’s view, these factors counseled in favor of its conclusion “that the [FDCA] and FDA regulations so thoroughly occupy the field of beverage labeling at issue in this case that it would be unreasonable to infer that Congress intended states to supplement this area.”
Finally, the District Court deferred to the agency’s expertise in the regulation of food and beverages. It asserted that it would be inappropriate for the Court to set rules, which the FDA “with all of its scientific expertise” has not yet done. Thus, the District Court concluded that the claims were “impliedly preempted” because “permitting states through statutes or common law causes of action to impose additional limitations and requirements on beverage labels such as described here would create obstacles to the accomplishment of Congress’s objectives….”
Holk filed this timely appeal…
III.
Snapple argues that the District Court’s dismissal must be upheld “whether analyzed under the doctrine of express preemption, implied `field’ preemption, or implied `obstacle’ preemption.” The preemption doctrine is rooted in Article VI of the United States Constitution, which states that the laws of the United States “shall be the supreme Law of the Land; … any Thing in the Constitution or Laws of any State to the Contrary notwithstanding.” U.S. Const. art. VI, cl. 2. Under the Supremacy Clause, federal law may be held to preempt state law where any of the three forms of preemption doctrine may be properly applied: express preemption, field preemption, and implied conflict preemption … We are guided in our preemption analysis “by the rule that `[t]he purpose of Congress is the ultimate touchstone in every preemption case.’”
…Additionally, we must begin our analysis by applying a presumption against preemption…“In areas of traditional state regulation, we assume that a federal statute has not supplanted state law unless Congress has made such an intention clear and manifest.” …This requires that, if confronted with two plausible interpretations of a statute, we “have a duty to accept the reading that disfavors pre-emption.”
Health and safety issues have traditionally fallen within the province of state regulation. This is true of the regulation of food and beverage labeling and branding…The federal government did not begin to regulate the labeling of food products until 1906, when Congress passed the Wiley Act. Nonetheless, Snapple argues that the presumption against preemption should not be applied “because of the century-long tradition of federal regulation over food and beverage misbranding, and the expansive scheme of juice-beverage labeling regulation in particular.” The Supreme Court, however, rejected a similar argument in Levine and applied the presumption…Accordingly, all of Snapple’s preemption arguments must overcome the presumption against preemption, as food labeling has been an area historically governed by state law.
A.
Snapple argues that Holk’s state law claims are expressly preempted by NLEA, specifically 21 U.S.C. § 343-1(a)(3). As a threshold matter, however, we must consider whether this issue is properly before us. As stated above, Holk initially argued that Snapple’s labels were misleading on several grounds, namely because Snapple claimed the products were “All Natural” despite containing HFCS and because Snapple advertised some products as containing juice that was not in the beverages. Holk subsequently dropped the argument related to the juice contents of certain Snapple beverages. This prompted Snapple to concede, during oral argument before the District Court, that it was no longer arguing express preemption: “[T]here’s only one preemption argument left because of the dropping of the juice claims…. There was expressed [sic] preemption, there was implied field preemption, and now there’s implied obstacle preemption. And it’s implied obstacle preemption that applies to the high fructose corn syrup natural claims.” Yet, on appeal, Snapple again raises express preemption.
Holk argues that because Snapple did not raise express preemption before the District Court in relation to her HFCS argument, Snapple has waived its express preemption argument. Snapple counters that “[w]here a new ground would support affirmance, this Court may invoke it so long as it is supported by the record.”
First, we note that the District Court did not rule in Snapple’s favor on express preemption. The Court stated that it “agrees with Plaintiff that Congress has not explicitly preempted Plaintiff’s claims by inserting any specific preemptive language into the [FDCA]….” It also noted that “Snapple’s express preemption arguments were directed at Plaintiff’s claims concerning the fruit juices contained in Snapple beverages, which Plaintiff has withdrawn.” Because the District Court did not rule in Snapple’s favor on its express preemption argument, we do not have an express preemption claim to affirm.
Second, Snapple is correct that this Court has held that “we may affirm a correct decision of the district court on grounds other than those relied upon by the district court.” … However, this rule does not apply to cases in which the party has waived the issue in the district court. This Court has stated: “We may affirm the lower court’s ruling on different grounds, provided the issue which forms the basis of our decision was before the lower court.”
…We conclude that Snapple has waived its express preemption argument with regard to Holk’s HFCS claims. Though Snapple contended in its two motions to dismiss that Holk’s juice content claims were expressly preempted by 21 U.S.C. § 343-1(a)(3), it did not raise this provision with regard to Holk’s HFCS claim. In fact, it did not raise any express preemption argument in response to the HFCS claim and explicitly disclaimed the applicability of express preemption to this claim. This clearly demonstrates that the issue was not before the District Court. For this reason, we conclude that the issue is waived.
B.
Field preemption occurs when state law occupies a “field reserved for federal regulation,” leaving no room for state regulation… It may also be inferred when “an Act of Congress `touch[es] a field in which the federal interest is so dominant that the federal system will be assumed to preclude enforcement of state laws on the same subject.’” …Nonetheless, for field preemption to be applicable, “congressional intent to supersede state laws must be `clear and manifest.’” … Snapple asserts that Holk’s claims are preempted because federal law occupies both the field of beverage regulation and the field of juice drinks regulation.
First, we note that NLEA declares that courts may not find implied preemption based on any provision of NLEA. It states that the Act “shall not be construed to preempt any provision of State law, unless such provision is expressly preempted under [21 U.S.C. § 343-1] of the Federal Food, Drug, and Cosmetic Act.” … Accordingly, if we are to find that Holk’s claims are impliedly preempted, we must do so based on provisions of federal law other than NLEA.