PGD and the making of the ‘genetic embryo’ as a political tool
3 PGD and the making of the ‘genetic embryo’ as a political tool
Martin H. Johnson and Anastasia A. Theodosiou
Introduction*
In 1990, the UK Parliament passed the Human Fertilisation and Embryology Act (HFEA 1990), which, among other provisions, legalised controlled research on human embryos. This Act resulted from six years of public and parliamentary debate, as well as lobbying for and against embryo research. Mulkay1 has discussed how the pro-research lobby made genetic disease a particular focus of the parliamentary debate.2 Pro-research lobbyists briefed MPs and the press, emphasising the potential to understand, prevent and even ‘greatly reduce the frequency of genetic disorders’.3 They drew particular attention to the desirability of diagnosing and selecting embryos based on their genetic characteristics, through the technique of pre-implantation genetic diagnosis (PGD), and contrasted this approach as being preferable to manipulating embryos by genetic engineering. Then, Robert Winston’s team announced the first clinical pregnancy following PGD to avoid transmission of sex-linked genetic disease just five days before the 1990 Commons vote.4 Mulkay suggests that this demonstration that research on human embryos really did produce genuine therapeutic benefits influenced parliamentary opinion decisively.5 Mulkay’s work offers a detailed sociological account of the tactics employed by the pro-research lobby between 1985 and 1990. He only hints, however, at the origins of these political arguments, as most of his discussion is based on the parliamentary debates and press commentary accompanying the passage of the HFEA 1990.
In this chapter, we explore how the concept of the ‘genetic embryo’ emerged as a political tool, and how the transformation of the embryo into a genetic entity affected the outcome of debate. While Mulkay suggests that genetic disease was only a ‘minor feature’ of the pro-research case until the late 1980s,6 we have argued recently that it played a critical role from as early as 1985 in structuring both research activities and Parliamentary debates preceding the passing of the HFEA 1990.7 In this chapter, we trace the history of the genetic embryo from its origins in the 1950s, through pioneering PGD studies in animals from as early as 1965, and in the discussions about prenatal testing and abortion. We suggest that the pro-research focus on genetic disease of the 1980s built on these earlier, often politically driven, discussions. We argue that the genetic embryo that ultimately emerged was a useful political tool, albeit one built on an insecure scientific foundation, and that its utility was due in part to exaggerated pro-research claims that went largely unchallenged.
The concept of the genetic embryo
We introduce the term ‘genetic embryo’ as a concept that was not simply employed as a political tool by the pro-research lobby, but rather was moulded by public, professional and Parliamentary perceptions of embryo research, in vitro fertilisation, PGD, abortion and prenatal testing. Our evidential sources include parliamentary debates and voting patterns, public opinion polls and media reports, scientific papers, and archival letters and documents. The phrase ‘genetic embryo’ was not actually used in these debates, but has been coined by us to encapsulate a variety of ways in which the genetic make-up of the embryo was a subject of debate and influenced the approach taken to the permissibility of scientific techniques. Use of this term provides a unifying conceptual framework within which to consider (a) the growing research and clinical interest in normal and abnormal genetics in human embryology, and (b) the geneticisation of political arguments in medico-scientific research policy and the 1980s’ UK embryo research debate. The concept of the genetic embryo can take a variety of forms, ranging from its use with reference to diagnosis, prevention or cure of genetic disease to simply understanding embryo genetics. Moreover, many references are vague about the technological details and social implications of the concepts described, perhaps due to insufficient information, or for strategic reasons. Nonetheless, the genetic embryo provides a common thread, which may be traced from the 1950s to 1990, allowing us to consider the evidence as to why the pro-research lobby came to focus strategically on genetic disease in the embryo research debate.
Early biomedical developments
The period after the Second World War saw the beginnings of the process by which genetics came to occupy its central position in biomedicine by the 1980s. A major practical stimulus to this early genetic research was to understand the impact of radiation-induced genetic damage to the germ line, which assumed particular political salience with the use of the atomic bomb in Japan, the radioactive fallout from atomic testing and the development of atomic energy.8 However, basic molecular and cytogenetic studies were to prove both complementary and, in the longer term, of more importance to the emergence of the genetic embryo. At the molecular level, the recognition that deoxyribonucleic acid (DNA) was the molecular carrier of genetic information9 was a key discovery, and was rapidly followed by the findings that each cell of the body carried a full set of DNA/genes10 that were selectively expressed as mRNA (messerge ribonucleic acid) to generate different cell phenotypes.11 Cytogenetic studies led to the acceptance of the human karyotype as having 46 chromosomes,12 agreement on the nomenclature of human chromosomes,13 and the discovery that chromosomal aneuploidies underlay developmental anomalies such as Down, Turner and Klinefelter syndromes.14 These latter discoveries led Robert Edwards to return to his pioneering 1950s’ studies on egg maturation in mouse and human,15 in an attempt to understand whether the causes of these aneuploidies might reside in abnormalities of meiotic maturation during oocyte development.16 Indeed, driven by this motivation, it was Edwards who first proposed the idea of PGD in 1965,17 and went on to demonstrate it in 1968.18
PGD and the genetic embryo
Using pre-implantation blastocysts flushed from the excised uteri of pregnant rabbits,19 Gardner and Edwards biopsied trophoblast and stained it for sex chromatin, providing a reliable method of sexing, which was compatible with further development of the embryo. Following transfer of the biopsied blastocysts to a pseudopregnant female, sex was confirmed anatomically and histologically at full term. Edwards and Gardner addressed the potential for clinical application explicitly, claiming that the scope of experimental embryology could be greatly extended to include the detection of ‘autosomally inherited deformities from either parent’.20
However, it was not until 1990 that the first clinical demonstration of PGD was achieved at the Hammersmith Hospital, London.21 Embryos from two couples were sexed, to exclude male embryos at risk of inheriting adrenoleukodystrophy or X-linked mental retardation. Unlike Edwards and Gardner’s study in rabbits, human embryos were obtained by IVF and sexed by polymerase chain reaction (PCR) amplification of DNA from a single 8-cell blastomere using Y-chromosome-specific probes.
Both of these demonstrations provided a method for sexing embryos without precluding subsequent development or transfer to a receptive uterus. However, due to species differences, methodological modifications were required before the work of Gardner and Edwards using rabbits could be applied clinically. Human embryos are more difficult to obtain practically and ethically than rabbit embryos, as well as being smaller and less amenable to manipulation. Thus, although human IVF was achieved experimentally in 1969,22 the first IVF baby was not born until 1978,23 and IVF was still widely viewed with suspicion until the late 1980s.24 Furthermore, while rabbit trophoblast biopsy provided 200–300 cells, biopsy of human embryos at the 8-cell stage yielded only one or two cells, necessitating far more sensitive genetic testing techniques. Such single-cell testing became theoretically possible in the late 1970s using micro-enzymatic assays,25 but these were not applied to PGD in mouse models for ten years26 and proved problematic in humans.27 It was not until the development of PCR in the late 1980s28 that single-cell testing of human embryos became a practical reality.29 It is these technological hurdles that have provided the conventional explanation as to why PGD took 22 years to be applied clinically.
However, we have argued recently that technological deficit offers an incomplete explanation for the somewhat protracted clinical development of PGD between 1968 and 1990.30 Thus, while there was very little discussion about, or experimental work on, PGD in humans before the mid-1980s, pre-implantation sexing for commercial incentives was pursued experimentally in cattle and large farm animals in the 1970s and early 1980s, although accuracy and feasibility were not tested on a large scale. Karyotyping, X-linked enzyme micro-assays and immunological detection of a male-specific antigen were each investigated as approaches to PGD that required neither PCR nor embryo biopsies of hundreds of cells.31 The dearth of human PGD studies in the 1970s and early 1980s suggests a relative lack of interest in overcoming the technological hurdles described. In contrast, the flurry of research and discussion seen between 1986 and 1990 suggests a sudden change in motivation to achieving clinical PGD, which we have ascribed to political events:32 notably the parliamentary opposition to research on human embryos that greeted publication of the Warnock Report.33 The potential application of PGD for the detection of genetic disorders became a critical part of the scientific response to this issue.
Abortion legislation in the 1960s: its role in the development of the genetic embryo
Just as the pro-research lobby in the latter half of the 1980s focused on the potential for embryo research to control genetic disease via PGD, so the 1960s’ pro-abortion parliamentarians’ debates on abortion legislation had highlighted the issue of detecting and terminating abnormal pregnancies. The potential for abortion to prevent the birth of handicapped children was one of two powerful arguments stimulating the passage of the Abortion Act 1967, the other being the desire to eliminate illegal and ‘back-street’ terminations in the interests of women’s health. In the wake of the thalidomide tragedy of the early 1960s,34 it was seen as ‘profoundly shocking’ that women were denied easily accessible, legal abortions ‘even in this extreme case when they knew their baby was likely to be born severely deformed’.35 At a time when doctors felt that they had ‘“failed to keep their contract” if the baby was anything but normal’,36 the widespread public and professional attention given to the thalidomide incident ‘enabled abortion to be formulated as a public health issue’.37
In July 1966, David Steel’s Medical Termination of Pregnancy Bill received a majority vote of 223:29 in the Commons. All 15 speakers made specific reference to Clause 1(b) of the Bill, which proposed allowing termination if there was a ‘substantial risk that if the child were born it would suffer from such physical or mental abnormalities as to be seriously handicapped’.38 Out of ten pro-abortion speakers, six accepted this clause ‘unreservedly’,39 arguing that the decision ‘should be left to the wishes of the mother and the judgement of her doctors’ so as to avoid the ‘suffering caused to both children and parents by such disasters’.40 One MP even shared personal experiences, defending his wife’s decision to terminate a pregnancy affected by rubella.41 Pro-abortion speakers cited the endorsement of Clause 1(b) by bodies such as the British Medical Association (BMA) and the Abortion Law Reform Association, as well as a national opinion poll stating that termination on ‘these grounds … received the highest support from public opinion – 91 per cent’.42 In contrast, four of the five anti-abortion speakers found Clause 1(b) to be ‘utterly repugnant’, reminding the House of ‘Hitler’s conception of a race of perfect physical specimens’,43 and questioning who should judge whether another ‘human being’s life is worth living’.44 The remaining four pro-abortion speakers and one anti-abortion MP took a more ambivalent stance, broadly approving of Clause 1(b), but warning of the ‘need for caution and for considerable discussion in Committee’.45
Aside from the argument of principle, one of the most significant sources of conflict in the 1960s’ abortion debates was whether or not foetal abnormality could be diagnosed accurately, or only estimated by risk assessment. Indeed, anti-abortion speakers insisted that such conditions were ‘not predictable’ or ‘diagnosable before birth’,46 while even pro-abortion speakers worried that ‘in none of these cases’ was there ‘anything approaching certainty’ that there would be ‘abnormality in the child’.47 Accordingly, parliamentarians referred far more frequently to risk than to diagnosis, and where the latter term was used, it was often to emphasise the woeful lack of diagnostic techniques (see Table 3.1). In the 14 debates on abortion between 1965 and 1967, there were only four references to amniocentesis, and no explicit references to ultrasound, as prenatal testing was not yet firmly established in clinical practice. As such, risk was mostly discussed in the context of exposure to infectious agents such as rubella, or teratogens like thalidomide, rather than genetic, chromosomal or hereditary factors (Table 3.1).
Table 3.1 Usage of key words in 14 Commons and Lords debates on abortion (1965–7)
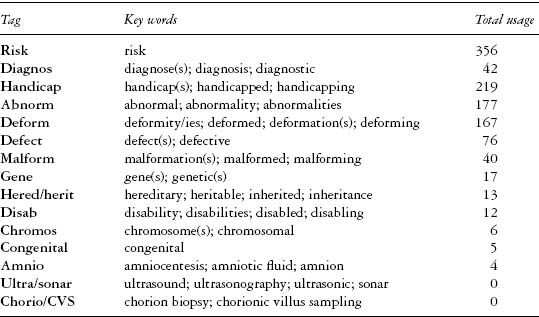
Note: Parliamentary rhetoric was analysed quantitatively by counting usage of key words electronically. For each of these key words, a suitable ‘tag’ was chosen to identify usage in different grammatical contexts (e.g. the tag ‘diagnos’ was used to identify the key words ‘diagnosis’, ‘diagnose(s)’ and ‘diagnostic’). A reference was counted only if spoken in the context of a genetic or non-genetic characteristic in an embryo or foetus.
Thus, the rhetoric of the genetic embryo evoked in the 1980s’ embryo research debates was preceded by Parliamentary reference to the ‘deformed foetus’ in the 1960s. There is evidence that the latter was very effective in securing the passage of the Abortion Act 1967. Despite uncertainty about the limits of medical diagnosis, it was agreed by a majority of 162:73 that abortion on grounds of ‘substantial risk’ of foetal abnormality should remain in the Bill,48 setting an important precedent of Parliamentary support for the principle of handicap prevention that was to influence the embryo research debates two decades later. Thus, in 1985, one pro-research speaker cited the Abortion Act 1967 as legal precedent, arguing that Parliament had ‘recognised the legitimacy of terminating the prospect of human life for a defective embryo’, and so it would be hypocritical to reject embryo research aimed at preventing such handicaps.49 Some MPs, like Renee Short, spoke in both the debates on abortion and on embryo research, emphasising in both cases the ‘heartbreaking and unaccountable tragedy’ of ‘congenital abnormality’,50 and the suffering of ‘unfortunate … disabled children’.51
Table 3.2 World Health Organization (WHO)-sponsored CVS registry
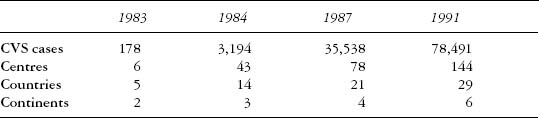
Source: based on Milunsky, Genetic Disorders and the Fetus: Diagnosis, Prevention, and Treatment, 3rd edn, Baltimore, MD: Johns Hopkins University Press, 1992, p. 124.
The advent of prenatal diagnosis
Although amniocentesis was pioneered to diagnose sex in 1955,52 prenatal testing did not become established in clinical practice until after the 1960s’ debates. The technique was subsequently expanded to enable diagnosis of dozens of metabolic and genetic disorders.53 Indeed, data collected by Guy’s Hospital indicated that the percentage of mothers aged 40 or more in their region who were tested increased from 12% in 1975 to 18% in 1976, 36% in 1977, and 42% in 1978.54 The clinical application of amniocentesis increased further following large-scale international clinical trials to assess safety in the late 1970s.55 Ultrasound was introduced into gynaecological and obstetric practice in the mid-1960s, and its clinical and experimental use rose rapidly throughout the 1970s,56 until it gained the approval of ‘virtually all prenatal diagnosticians’.57 In the mid-1980s, first trimester chorionic villus sampling (CVS) began to gain clinical popularity (see Table 3.2), as it provided an earlier alternative to second trimester amniocentesis followed by later, and thus more physically and emotionally traumatic, abortions.
By the 1980s, these developments had largely naturalised the concept of intervening in pregnancy to prevent abnormality. Indeed, in 1982, while National Health Service (NHS) clinics performed only 29% of the abortions that took place after 17 weeks (the remainder being carried out by private practitioners), they performed 85% of the late abortions for suspected foetal abnormality.58 These figures indicate ‘the reluctance of most gynaecologists to carry out late abortion[s]’, but also the ‘special status given to congenital abnormality’.59 By 1989, the Royal College of Physicians reported that 60–85% of ‘informed women at risk’ requested amniocentesis in the UK, and that, in areas where screening was routine, the birth rate of babies with neural tube defects had fallen by 60–70%.60 As such, during the embryo research debates of the 1980s, even many opponents of embryo research wanted ‘to see diseases and handicaps eliminated’,61 and were ‘at least equally concerned to find cures’ as were the pro-research speakers,62 objecting to the need for destructive embryo research rather than the principle of intervening to select genetically healthy embryos for transfer. Thus, the political and clinical experience with abortion on grounds of handicap prevention from the 1960s onwards likely contributed to the political status and effectiveness of the concept of the genetic embryo in the 1980s.
The genetic embryo and the Medical Research Council
While prenatal testing was becoming increasingly acceptable clinically during the 1970s, the potential to control genetic disease using PGD was initially overshadowed by widespread scepticism and ethical concerns about IVF and embryo research. Indeed, the Medical Research Council (MRC) found research on or transfer of IVF embryos to be unethical, and refused to fund such research until ‘they were provided with satisfactory evidence that there would be no increased risk of abnormal offspring’.63 In 1971, the MRC considered and ultimately rejected a grant application by Robert Edwards and Patrick Steptoe to fund their research on human conception and development in vitro, one objective of which was to facilitate development of PGD.64 Johnson et al. provide a detailed analysis of this decision, explaining that the Council viewed IVF as ‘purely experimental rather than as potentially therapeutic, and so set the bar for safety high’. 65 Grant referees John Evans and Roger Short rejected the possibility that embryo sexing could avert the birth of children with sex-linked defects, and found amniocentesis followed by abortion to be ‘far simpler’ and even ‘less hazardous both for the mother and the foetus’.66 The MRC advocated experiments on non-human primates, but added that only the ‘eventual birth, after transfer, of a normal baby’ could satisfy them that IVF did not cause abnormalities.67 Even Anne McLaren, who later played a central role in the pro-embryo-research lobby, publicly expressed concern that ‘a malformed baby’ might be born if transfers were ‘carried out prematurely, through the desire … to be first in the field’.68
In contrast, Edwards and Steptoe were among the first to recognise the scope for embryo research to prevent, rather than cause, genetic abnormalities. Indeed, they attempted to biopsy and sex human blastocysts in the early 1970s, albeit without success,69 and suggested that this would be ‘an excellent approach to the control of sex-linked mutant genes in man’.70 In their 1971 grant application to the MRC, they acknowledged research into ‘averting the birth of children with certain inherited disorders’ as one of their core objectives, along with the ‘alleviation of infertility’.71 These aims were reiterated in a working party report of the British Association for the Advancement of Science,72 of which Edwards was a member. In addition, a 1972 study by the BMA reported on the potential to ‘diagnose certain foetal abnormalities in fertilised ova’, and that ‘this way might be preferable to termination at 16 weeks’ following amniocentesis.73
The birth of the first IVF baby in 1978, free from apparent congenital abnormality, ‘vindicated Edwards and put pressure on the MRC to justify and reverse a decision that now looked wrong’.74 While the MRC had previously given ‘infertility a low priority compared to population control’,75 the clinical success of IVF ‘transformed’ the public’s ‘latent demand for a technological solution to the problem of childlessness into an active demand’ which the Council could not ignore.76 In October 1978, the MRC advised ‘an urgent review’ of policy, ‘in light of the recent demonstration of the feasibility of human in vitro fertilisation and embryo transfer, and proposals received by the Cell Board both from Dr Anne McLaren … and from Professor R V Short’.77 The statement from Short, however, referred only to his desire to use ‘human spermatozoa to fertilize … hamster eggs’ in order to study anomalies in haploid sperm nuclei,78 while McLaren’s contribution was somewhat ambivalent. Although she concluded that a policy review ‘would be no bad thing’, she did not ‘see any great urgency’ at that time,79 which implies that the Council’s decision to review their previous policy was more influenced by the birth of a healthy IVF baby than by the expert opinion received. However, McLaren did caution that ‘we need to be certain’ that IVF embryos ‘are not abnormal’, and stressed that ‘the only ethical approach’ would be ‘to carry out chromosomal and biochemical studies’ on pre-implantation embryos, which ‘might well also throw light on the aetiology of spontaneous abnormalities of implantation and early development’.80 Critically, she specified that ‘there would be no question’ of research embryos being transferred, and so ‘no increased risk of abnormal offspring’.81 Thus, while the fear that IVF might cause anomalies remained, it was suddenly used as a justification to encourage, rather than withhold, support for research on human embryos.
Following the MRC’s decision, an MRC Advisory Group met in March 1979, made up largely of reproductive physiologists and clinicians.82 They concluded that ‘scientifically sound research’ should be allowed, provided that the aim was ‘clearly defined and acceptable’, and ‘relevant to clinical problems such as contraception or the differential diagnosis and treatment of infertility’.83 Although they did not specify the study of inherited disease in these conclusions, they did note ‘with interest the recent developments of the use of recombinant DNA techniques in the field of prenatal diagnosis … which might become relevant’ to the ‘prevention of inherited disease’. The Advisory Group met again in May 1982, and this time agreed that ‘all the available evidence in animals’ suggested that there were ‘no adverse effects on offspring’ born by IVF.84 Based on this opinion, the MRC, for the first time, encouraged the pursuit of ‘a screening device for determining the chromosome constitution of an embryo’ in order to avoid genetic disease. This perspective on PGD was offered by none other than John Evans, who, in 1971, had deemed Edwards’ suggestion of the same technology to be ‘minor’, ‘complicated and unnecessary’.85 The increased focus on embryo genetics was reflected in the Advisory Group’s expanded membership, which now included the genetics expertise of McLaren, Evans, Polani and Williamson. The Group reiterated that research should be allowed if ‘relevant to clinical problems such as contraception and … infertility’, but wished ‘to include … the fields of embryology and inherited disease’.86 That November, the Council published these guidelines in the British Medical Journal,87 providing an important public endorsement of embryo research by a professional scientific body, and formalising the tripartite conception of research to tackle contraception, infertility and genetic disease.
Some of the earliest embryo research projects supported by the MRC focused on this new third aim, including work by Angell et al.88 at the MRC’s Reproductive Biology Unit in Edinburgh. This study investigated the ‘high failure rate (80%) of implantation’ in clinical IVF, and contributed the ‘surprising and remarkable finding’ that 3/11 embryos tested showed chromosomal defects. They concluded that such defects ‘clearly result in early embryo loss’,89 and so used genetic arguments to justify the need for further research to improve the success rate of IVF. Johnson, Braude and Pratt received the first MRC external grant supporting human embryo research in 1983, which aimed ‘to draw on the knowledge of the mouse embryo to establish criteria of normal development in the human embryo, and to apply these to detect causes of abnormality and reproductive failure’.90 These individuals were heavily involved in pro-research lobbying from the mid-1980s onwards, making them well placed to publicise the potential for embryo research to facilitate detection and control of genetic disease.
Thus, before 1978, limited references to the concept that we have termed the genetic embryo were eclipsed by fears that IVF would cause, rather than prevent, abnormalities. However, the notion of the genetic embryo took shape in medico-scientific rhetoric from 1978 onwards, and was used by the MRC to justify a radical policy change on human embryo research. In this way, the fear of elusive IVF abnormalities was transformed into a desire to pursue, rather than impede, research on genetic abnormalities in IVF embryos.
The genetic embryo and the Warnock Committee
In July 1982, the Department of Health and Social Security (DHSS) formed a Committee of Inquiry to investigate ‘the social, ethical and legal implications’ of ‘recent and potential developments in medicine and science related to human fertilisation and embryology’,91 chaired by Dame (now Baroness) Mary Warnock. A Government circular was issued in late 1982 by Jenny Croft, one of the Committee’s two Secretaries, calling for submission of evidence by numerous medical, scientific, religious, charity and lobby groups.92 The circular requested evidence specifically on the ‘therapeutic … scientific … legal … social … moral and ethical’ implications of ‘recent and potential developments’ in human fertilisation and embryology, and identified the ‘therapeutic aspects’ as involving ‘the treatment of infertility and the prevention of inherited disease’.93 An explanatory note was also attached, which outlined the technological basis of assisted reproduction, and discussed the potential for research on embryo genetics involving trans-species fertilisation, sexing, genetic manipulation and cloning ‘to investigate the chromosome normality’.94 As such, the genetic embryo was framed as an important player, although not yet the main protagonist, in this circular, which may have influenced the focus of the evidence submitted subsequently. Jeremy Metters, the Committee’s other Joint Secretary, may have contributed to this framing, as he was an observer at the 1979 and 1982 MRC meetings on policy review,95 and so may have been influenced by the MRC’s increasing focus on the genetic embryo.
We have reviewed the evidence submitted by six major medico-scientific bodies, including the MRC,96 the BMA,97 the Royal College of Obstetricians and Gynaecologists (RCOG),98 the Royal Society,99 the Royal College of General Practitioners (RCGP)100 and the Council for Science and Society (CSS).101 With the exception of the RCGP, all were broadly pro-research. However, they differed somewhat in focus, with the reports of the MRC and the Royal Society devoted entirely to the practicalities and wider implications of embryo research, while the evidence by the BMA and the RCGP was focused more on the ethics and provision of infertility treatment. Nonetheless, almost all of these bodies identified the control of genetic disease as one of the main potential benefits of research, alongside infertility treatment. Alternative approaches to PGD were discussed, such as ‘the removal of cells from the very early embryo’,102 or ‘embryo division’,103 and the reports advised that intact embryos could be frozen for later transfer, while the cells or ‘cloned’ embryos could be sexed to exclude sex-linked disease, or tested using ‘DNA probes’.104 Significantly, these suggestions preceded the technological developments, such as PCR, which facilitated research progress on human PGD from 1986 onwards.105
It was proposed that such methods were ‘more acceptable than amniocentesis with a view to mid-trimester termination’,106 and that, while IVF treatment would ‘be of help only to that small percentage of parents who are ever likely to use the IVF procedure’, a ‘better understanding of inherited disease … could be of immense benefit to … vast numbers of individuals’.107 Furthermore, these medico-scientific bodies either condemned ethically challenging aspects of research on embryo genetics, such as sexing for non-medical reasons,108 or else downplayed these aspects. In a presentation to the Warnock Committee, the MRC stressed that genetic manipulation of embryos was not ‘a simple or even desirable method of preventing genetic disease’,109 while the CSS insisted that ‘Aldous Huxley’s literary fantasy’ of reproductive cloning was ‘very far indeed from realization’.110 As such, the pro-research rhetoric, identified by Mulkay111 as being pivotal to the passage of the HFE Act 1990, featured already in the evidence of a handful of major medico-scientific bodies in 1982/3. In addition, several individuals involved in producing this evidence subsequently participated heavily in pro-research lobbying, such as Anne McLaren and M. C. Macnaughton, and so were well placed to re-emphasise such rhetoric. Thus, the genetic embryo was emerging as a political tool significantly earlier than Mulkay suggests.
Considering that the Committee’s call for evidence112