Misbranding
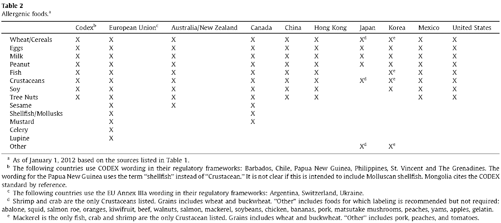
Fig. 4.1
FDA’s mission
4.1.2 Overview of Constitutional Challenges
Misbranding actions strike at some of the most closely held constitutional rights. Limitations on labeling impact both a facility’s First Amendment right to free speech and the consumers freedom of choice. Constitutional rights stand in equal, if not greater, importance to the FDA’s statutory mission to protect the public from fraudulent labeling.
The constitutional question often involves definitional questions. The central issue often involves what constitutes labeling. It is this reach by the FDA to regulate in new areas that consumes many of the judicial challenges. Constitutional challenges rarely feature the misbranding concept. Other questions arise, such as what type of speech is made on a label. The varying options, regulated, commercial, or ordinary, all carry case law and criteria that determine the outcome of the challenge.
Some constitutional challenges do not involve the FDA’s attempt to take enforcement action. In fact it is often the FDA’s inaction that prompts attempts to regulate misbranding or labeling provisions in the market. Often, this is seen where a competitor is making putatively violative claims that offer it an unfair advantage. This constitutional case involves the issue of preemption and whether a private party can take on the enforcement activities of the FDA. Recent Supreme Court case law informs much of the discussion on this topic.
Balancing these three opposing interests sets the boundaries on permissible enforcement actions. The FDA can take enforcement actions where it does not interfere with a constitutional right or freedom. Likewise, the FDA’s authority proscribes some private enforcement actions. Where the three competing interests are balanced a valid misbranding grievance exists (Fig. 4.2).
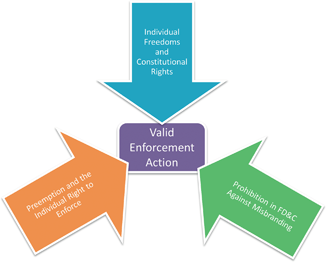
Fig. 4.2
Competing constitutional and statutory interests
4.1.3 The USDA and Misbranding
At first blush many of the definitions used under the PPI and FMI will look similar to those used by the FDA. Both the FMI and PPI define “label” and “labeling” in nearly identical terms to those used by the FD&C. FSIS does, however, employ a much longer list of acts that constitute misbranding.
FMI Sections 601(o) and (p) and PPI Section 451 (s)
A label is “a display of written, printed, or graphic matter upon the immediate container of any article.”
Labeling is “all labels and other written, printed, or graphic matter (1) upon any article or any of its containers or wrappers, or (2) accompanying such article.”
Misbranding Circumstances
FMI Section 601(n) and PPI Section 451 (h)
1.
if its labeling is false or misleading in any particular;
2.
if it is offered for sale under the name of another food;
3.
if it is an imitation of another food, unless its label bears, in type of uniform size and prominence, the word “imitation” and immediately thereafter, the name of the food imitated;
4.
if its container is so made, formed, or filled as to be misleading;
5.
if in a package or other container unless it bears a label showing (A) the name and place of business of the manufacturer, packer, or distributor; and (B) an accurate statement of the quantity of the contents in terms of weight, measure, or numerical count: Provided, That under clause (B) of this subparagraph (5), reasonable variations may be permitted, and exemptions as to small packages may be established, by regulations prescribed by the Secretary;
6.
if any word, statement, or other information required by or under authority of this chapter to appear on the label or other labeling is not prominently placed thereon with such conspicuousness (as compared with other words, statements, designs, or devices, in the labeling) and in such terms as to render it likely to be read and understood by the ordinary individual under customary conditions of purchase and use;
7.
if it purports to be or is represented as a food for which a definition and standard of identity or composition has been prescribed by regulations of the Secretary under Section 607 of this title unless (A) it conforms to such definition and standard, and (B) its label bears the name of the food specified in the definition and standard and, insofar as may be required by such regulations, the common names of optional ingredients (other than spices, flavoring, and coloring) present in such food;
8.
if it purports to be or is represented as a food for which a standard or standards of fill of container have been prescribed by regulations of the Secretary under Section 607 of this title, and it falls below the standard of fill of container applicable thereto, unless its label bears, in such manner and form as such regulations specify, a statement that it falls below such standard;
9.
if it is not subject to the provisions of subparagraph (7), unless its label bears (A) the common or usual name of the food, if any there be, and (B) in case it is fabricated from two or more ingredients, the common or usual name of each such ingredient; except that spices, flavorings, and colorings may, when authorized by the Secretary, be designated as spices, flavorings, and colorings without naming each: Provided, That to the extent that compliance with the requirements of clause (B) of this subparagraph (9) is impracticable, or results in deception or unfair competition, exemptions shall be established by regulations promulgated by the Secretary;
10.
if it purports to be or is represented for special dietary uses, unless its label bears such information concerning its vitamin, mineral, and other dietary properties as the Secretary, after consultation with the Secretary of Health and Human Services, determines to be, and by regulations prescribes as, necessary in order fully to inform purchasers as to its value for such uses;
11.
if it bears or contains any artificial flavoring, artificial coloring, or chemical preservative, unless it bears labeling stating that fact: Provided, That, to the extent that compliance with the requirements of this subparagraph (11) is impracticable, exemptions shall be established by regulations promulgated by the Secretary; or
12.
if it fails to bear, directly thereon or on its container, as the Secretary may by regulations prescribe, the inspection legend and, unrestricted by any of the foregoing, such other information as the Secretary may require in such regulations to assure that it will not have false or misleading labeling and that the public will be informed of the manner of handling required to maintain the article in a wholesome condition.
The USDA follows an entirely different model of policing misbranding. Whereas the FDA allows products to enter the market before taking enforcement action, FSIS preapproves every label used prior to a product entering the market. The difference is in part due to the statutory language in the FMI and PPI. In Sections 607(d) and 457(c) the statute states products may only be sold that are not misleading and which are “approved by the Secretary” of the USDA. This “approved by” language is absent from the FD&C. It is also the textual hook the USDA interprets as mandating preapproval of all food labels before the products are offered for sale.
FMI Sections 607(d) and PPI Section 457(c)
(d) Sales under false or misleading name, other marking or labeling or in containers of misleading form or size; trade names, and other marking, labeling, and containers approved by Secretary
No article subject to this subchapter shall be sold or offered for sale by any person, firm, or corporation, in commerce, under any name or other marking or labeling which is false or misleading, or in any container of a misleading form or size, but established trade names and other marking and labeling and containers which are not false or misleading and which are approved by the Secretary are permitted.
The full ramifications of FSIS’s authority can now be seen. In Chapter 2 the text outlined FSIS’s unique continuous compulsory inspection model. In labeling, FSIS exercises similar control with preapproval for all labels. FSIS is given much stricter controls to ensure meat and meat products that enter the market are both safe and genuine ( See Fig. 4.3).
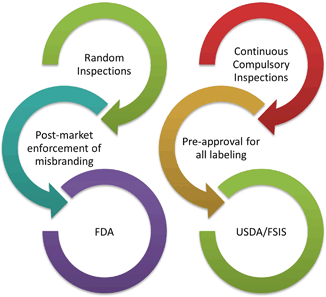
Fig. 4.3
Comparing FDA and FSIS enforcement models
FSIS attempts to ease the potential preapproval burden using two tools. Prior approval is granted in one of two ways. FSIS will either provide a sketch approval or generic approval. Sketch approval involves a review by the Labeling and Program Delivery Staff whereas generic approval merely requires compliance with applicable regulations. FSIS regulations approve some labels without submitting a sketch or concept label . Although it appears FSIS is abandoning its prior approval labeling mandate by using its generic approval system it technically continues to approve all labels. Akin to continuous inspection taking on one meaning for slaughter facilities and another for processing plants, prior approval can be achieved in a variety of ways. FSIS holds that prior approval is maintained either by FSIS staff reviewing draft labels or FSIS develops regulations that a facility must comply before entering the market. Thus, it can be said all FSIS labels are preapproved.
4.2 Defining the Label
4.2.1 When Written Material Becomes Labeling
Independent Books and Literature
One of the key threshold questions when discussing FDA enforcement of misbranding is distinguishing labeling from advertising. This will inform the gamut of statutory and constitutional issues raised by the FDA, industry, or the courts. In the absence of a clear sense of whether the written material in question is labeling or advertising, there is no indication what statutory criteria is to be applied in evaluating misleading or misbranding. This is of particular importance given the concurrent jurisdiction exercised by the FTC over advertising.
The statutory definition itself is intentionally broad and flexible. Nearly any written materially associated with a food product can be captured under Section 201(k). In addition, 201(m)(2) carries a textual hook, “accompanying such article,” that would allow for easy expansion of the concept. rather than freezing the label concept to the period of the Act, Congress sought to provide the FDA flexibility to keep pace with labeling and marketing practices. The definition gains new elasticity when courts abandon the strict construction standard for interpreting the Act.
Courts interpret the Act broadly. As discussed in Chapter 1 courts routinely cite the drafter’s intent to interpret the Act broadly. Courts also find a liberal construction consistent with the Act’s intent to protect the public from harm. Whether that harm is physical or economic is immaterial. Thus, an expansive definition meets little resistance from courts reviewing the FDA’s efforts to stretch the concept.
One of the first cases to consider what constituted labeling found literature written by an independent author as labeling. The case, United States v. 250 Jars ‘Cal’s Tupelo Blossom U.S. Fancy Pure Honey’, was decided by the Sixth Circuit in 1965.1 In Cal’s Honey the court reviewed a misbranding of the retail store of Cal’s Tupelo Honey displayed with copies of a booklet titled “About Honey” ( Cal’s Tupelo Honey).2 The booklets were placed on the shelf above the honey. An FDA inspector entered the store acting as customer and requested information about honey. the clerk handed the undercover agent a newsletter containing an article “Eat Honey and Increase Your Vitality” ( Cal’s Tupelo Honey).3 This was apparently the common practice of the store. The FDA seized 71 copies of the newsletter from the store. The newsletter contained a number of statements about the benefits of eating honey. This included a claim that the honey was a “panacea for various diseases and ailments that have plagued man from time immemorial” ( Cal’s Tupelo Honey).4 The store also mailed the newsletter to prospective clients as a type of direct-mail marketing practice.
The court found the book and newsletter as labeling based on the connection to the retailer’s products. The court began by reasoning the misbranding provisions of the Act were to be liberally construed because it was “passed to protect unwary customers in vital matters of health…” ( Cal’s Tupelo Honey).5 From this lens the court concluded it must uphold the action against the retailer or risk opening a “…loophole through which those who prey upon the weakness, gullibility, and superstition of human nature…” ( Cal’s Tupelo Honey).6 This combined with the “immediate connection” of the literature and the sale of the products resulted in a finding that the independent materials were part of the food label or labeling. Thus any written material used to effectuate the sale constitute labeling under the Act.
United States v. 250 Jars ‘Cal’s Tupelo Blossom U.S. Fancy Pure Honey’
McALLISTER, Senior Circuit Judge.
The United States brought a libel proceeding for the condemnation, under Section 304(a) of the Federal Food, Drug and Cosmetic Act, as amended, Title 21, U.S.C.A. § 334(a) of a quantity of allegedly misbranded honey, sold by claimant-appellant at one of its stores located in Detroit, Michigan.
Upon stipulated facts, and after argument, the District Court entered an order of condemnation for misbranding of the honey, from which claimant appeals.
It appears that the honey which was condemned was displayed on shelves in appellant’s retail store, on top of which were placed copies of a booklet, “About Honey,” which were sold to any customer desiring to purchase them. On the store premises, when the honey was seized, were 71 copies of a newspaper-type mailing piece, containing an article “Eat Honey and Increase Your Vitality.” The booklet and the newspaper leaflet were shown to a drug inspector, acting as a prospective customer, in response to his request for information about honey. The information contained in the foregoing publicity material was also mailed to prospective customers in order to promote the sale of the honey.
Appellant contends that the booklet, “About Honey,” by an independent author, which was on sale in the Book Department of its retail store, and the newspaper leaflets located in a back room of the store, did not constitute misbranding; and that the inferences from the evidence relied upon by the District Court were negated by uncontroverted facts.
Judge Freeman found that the booklet and the newspaper leaflet constituted labeling and misbranding of the honey, and that a reading of the booklets and mailing leaflets resulted in the inescapable conclusion that such honey was intended to be used as a drug, since the literature made the rather remarkable claim that honey is a panacea for various diseases and ailments that have plagued man from time immemorial. Furthermore, Judge Freeman held that the Act was passed to protect unwary customers in vital matters of health and, consequently, must be given a liberal construction to effectuate this high purpose, and that the Court would not open a loophole through which those who prey upon the weakness, gullibility, and superstition of human nature can escape the consequences of their actions. Upon a review of the record and the briefs of the parties, we are in accord with the trial judge’s reasoning and his conclusion.
It should here be observed that, since the determination of the District Court in this case, there has been decided by the Court of Appeals for the Second Circuit, a case involving the sale of food products in a store in which misleading books, relating to food, were also sold, and a judgment of condemnation by the District Court in that case, was reversed. However, that case differs from the one before us in that, although misleading books were sold in the same store that sold the product which was seized and condemned, there was no immediate connection of the misleading books with the sale of the product, and, as the court said, the books were not put to such use by the seller of the product.
As we have said in prior cases, it is not the policy or practice of this court, in reviewing cases on appeal where a District Court has rendered a comprehensive opinion with which we find ourselves in full agreement, to rewrite such an opinion and, in a sense, deprive the trial court of the credit of its careful consideration of the issues and arguments, and complete determination of the cause.
In accordance with the foregoing, the judgment of the District Court is affirmed for the reasons set forth in the opinion of Judge Freeman…
Comparing the decision in Cal’s Honey to two other cases provides a clearer sense of when independent literature constitutes labeling. In district court case preceding Cal’s Honey a maker of blackstrap molasses closely coordinated the promotion of its product through a book written by an independent author ( Plantation Molasses).7 The maker of the blackstrap molasses placed copies of the book along with jars of the molasses in the store window. With the display was a sign stating customers could order all the products required by the “Hauser diet” including blackstrap molasses ( Plantation Molasses).8 The sign invited customers in with the sign stating “Come in for full information” ( Plantation Molasses).9 A customer intrigued by the sign and inquiring about the diet would be handed a copy of the book and directed to the pages discussing the use and benefits of blackstrap molasses ( Plantation Molasses).10 In Cal’s Honey the book was placed near the honey or mailed to customers, but was never overtly marketed like the book on the Hauser diet.
Yet simply having books in the same store is not enough to constitute labeling. The Second Circuit reviewed a case around the time the Sixth Circuit reviewed Cal’s Honey. The case focused on a condemnation ruling against Balanced Foods, Inc., a maker of sterling cider vinegar and honey ( Sterling Vinegar Honey).11 Balanced foods carried its products and two books, “Folk Medicine” and “Arthritis and Folk Medicine” along with other products ( Sterling Vinegar Honey).12 The books, vinegar and honey were seized by the FDA from its warehouse in New York. The district court found the books were labeling and condemned the “labeling” as misbranded ( Sterling Vinegar Honey).13 The books promoted the use of sterling cider vinegar as suitable for medicinal use ( Sterling Vinegar Honey).14 The products itself made no claims other than the contents of the bottle. The Second Circuit provides the first boundary on what constitutes labeling. It reasoned:
On the other hand, labeling does not include every writing which bears some relation to the product. There is a line to be drawn, and, if the statutory purpose is to be served, it must be drawn in terms of the function served by the writing ( Sterling Vinegar Honey).15
The court held that there must be some “immediate connection” for written material to constitute labeling, such as joint promotion ( Sterling Vinegar Honey).16 In assessing joint promotion, the court looked at evidence of shelving together, proximity of the products, and displays featuring the books and the products ( Sterling Vinegar Honey).17 Merely carrying two related products was insufficient to function as a connection between the products (Fig. 4.4).
Fig. 4.4
Illustration of factors weighed when assessing whether independent literature is labeling
Images, Social Media, and Other “Labeling” Materials
The general framework that emerges from judicial precedent is that any material that bears a strong relationship to the sale of the product is labeling. Further, case law clarifies the FDA’s policy on when images, website material, social media and metadata constitute labeling. In both Cal’s Honey and Plantation Molasses the court focused on whether the written material “accompanies” the food product. This lead the court to conclude that any immediate connection would mean the written material accompanied the food product. An earlier case provides another definition to “accompanies.”
The Supreme Court reviewed the question of when labeling accompanies a product in the drug context. In 1948 the Supreme Court decided Kordel v. United States.18 Kordel involved a drug company mailing drugs and explanatory pamphlets to its retailers in separate packages often at different times. The pamphlets explained the benefits and effectiveness of the drugs, which Kordel wrote based on private and public research. The court found it was not necessary for the written material to physically accompany the products ( Kordel).19 Instead “accompanies” refers to a textual relationship between the written material and the product. When written materials “supplements or explains” the product, then it accompanies it ( Kordel).20 This decision along with Cal’s Honey and Plantation Molasses form the basis of the FDA’s policy to sweep-in a broad range of marketing materials as labeling.
The FDA’s policy is far reaching. In policy statements for every regulated category the FDA cites to Kordel. Under Kordel it interprets “labeling” as defined in the Act to include brochures, booklets, video, sound, images, websites, social media, and metadata. Courts continue to agree. The central question to determine whether material constitutes label is not its form, but whether it “accompanies” the regulated product. To accompany the regulated product the material must “supplement or explain” it ( See Fig. 4.5).
The FDA does not always meet the supplements-or-explains standard. It may seem defeating to read the case law giving the FDA copious coverage to deem virtually all material labeling. Still there are instances where the courts limit the labeling concept. For example, a district court in 2013 dismissed an attempt to bring a false advertising case against Frito-Lay ( Frito-Lay North America).21 The plaintiffs sought to include Frito-Lay’s website as part of the company’s label. It cited the language, “www.fritolay.com,” found on the packaging as evidence of integrated marketing ( Frito-Lay North America).22 The court disagreed that the website was enough under Kordel:
The Court does not find that the language on the www.fritolay.com website constitutes labeling under the FDCA, because as cited by Plaintiffs, none of the website language explains or supplements the individual Named Products such that the website could generally be found to “accompany” the Named Products. Even though the Named Products’ labels ask consumers to visit the website, they do not state that the website will inform consumers of the details of the Named Products’ nutritional facts, and none of the language Plaintiffs cite is drawn closely enough to the Named Products themselves to merit the website’s being found to constitute “labeling” (emphasis added).23
Listing a website is commonplace in the market today. The district court found how the website is listed matters as much as what is on the website. Frito-Lay simply listed the website and did not add language like “learn more” or “get more nutrition information” to suggest the website supplemented or explained the product. Also, the website was fairly generic. It contained photos and company information, but no specific statements that could be construed as explaining or supplementing the information on the package. Together the evidence could not support the supplements-or-explains standard provided in Kordel.
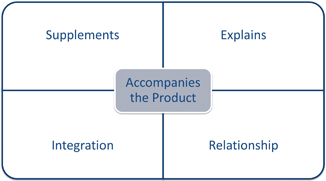
Fig. 4.5
Illustration of Kordel criteria
4.2.2 Regulatory Components of a Label
In addition to the global concept of what constitutes labeling is the more focused regulatory question on the regulatory required components of labeling. The components discussed here fit the traditional notion of labeling relating specifically to product packaging. Under the regulations of food labeling two chief packaging areas are defined, each carrying certain information and disclosures.
The principal display panel is a regulatory name for the well-known face of the package. It is the main label panel either facing out from the shelf or alternatively at the top of packaging. The CFR defines the principal display panel or PDP as “that portion of the package label that is most likely to be seen by the consumer at the time of purchase” (21 CFR Part 101).24 The PDP must contain the name of the food, known as the statement of identity, and the net quantity statement ( See Fig. 4.6). This sounds technical, but is seen every day when we shop in a market or examine the cupboard.

Fig. 4.6
FDA labeling guidance identifying PDP and net quantity statement
The information panel is another regulatory term for an intimately familiar part of the packaging. The information panel is the panel that contains the nutrition facts and ingredients listing ( See Fig. 4.7). The regulations require the information panel “immediately to the right of the PDP, as displayed to the consumer” (21 CFR Part 101).25 If the packaging does not accommodate useable space immediately to the right of the PDP then the next label immediately to the right suffices (21 CFR Part 101).26 The information panel cannot contain any “intervening material” with solely the required name and address of the manufacturer, packer or distributor, the nutrition labeling, any required allergy labeling, and the ingredient list (21 CFR Part 101).27
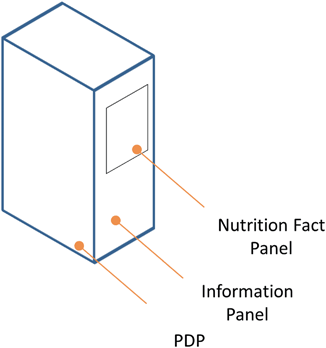
Fig. 4.7
Illustration identifying location of information panel
The regulations contain a wealth of detail covering every facet of the packaging. This includes the types of information required, the location, and in some cases even the size of font used. Understanding this element of labeling remains a critical task for daily operations and compliance.
4.3 What Constitutes Misbranding
Equipped with an understanding of what is a “label” or “labeling” the focus shifts to when that label violates the Act. This will involve a review of the full range of written material from social media to product packaging including images and video material.
There are several grounds for finding an FDA-regulated product misbranded. This section will discuss the most common violations as well as provide coverage of unique areas of labeling, such as allergens, “all natural” claims, and organic. Irrespective of the category or type of misbranding the overall prohibition is against labeling material that is misleading. As will be seen there is a grand gradient of mislabeling from the missing regulatory components to the egregious claims about product efficacy or benefits.
4.3.1 Misleading Labels
The central statutory prohibition for all labeling is against misleading labels. To protect the public the overarching aim of labeling enforcement is against misleading labeling. Labeling is one aspect of food law consumers are all familiar with. We all exhibit our own product preferences and interact with the label to assess its attributes, in particular products new to our pantry. Some will hone in on the ingredient list others immediately look for hallmark claims such as “all natural” or “high in fiber.” This practice builds in a misleading-radar. As consumers we can all assess the credibility of labeling and marketing claims to varying degrees. This section will fine-tune that misleading-radar to pickup the nuances of statutory provisions and case law interpretations.
Starting with the FD&C a broad prohibition is laid down. Section 201 broadly proclaims food is deemed misbranded if misleading. It goes on to provide some criteria for finding misleading, including omitting material facts about the consequences of using the product. Otherwise the statute provides no indication of when a label is misleading and thus misbranded . This is known as the “materiality analysis” whereby omitted facts are evaluated to determine if they were material facts. The plain meaning of misleading encompasses more than factual omissions.
Misleading nutritional information qualifies for misbranding under Section 201(n). This was seen in United States v. An Article of Food… Nuclomin ( Nuclomin ).28 The Eighth Circuit reviewed on appeal a vitamin/mineral tablet known as “Nuclomin.” The FDA deemed the supplement misbranded and seized it ( Nuclomin).29 As will be discussed in Chapter 6 dietary supplements are regulated as food products. The district court concluded many of the ingredients listed on the label were of no nutritional value or at such “minute” levels as not to offer a nutritional value to the supplement ( Nuclomin).30 On appeal the Eight Circuit found “substantial evidence” that the disputed ingredients in Nuclomin were either “not needed in the human diet” or the levels were so small as to offer no value to the consumer ( Nuclomin).31 Although technically compliant by listing all of the dietary ingredients the ingredients could “persuade a purchaser that the product possessed greater nutritional value” than it or its competitors contained ( Nuclomin).32
The FDA is not required to present evidence that consumers were misled. The maker of Nuclomin argued on appeal that the FDA failed to prove any of its customers were actually misled. The Eight Circuit cited to prior precedent holding that the “fact that no purchasers have actually been misled is not a defense under the Act” ( Nuclomin).33
United States v. An Article of Food… Nuclomin
LAY, Circuit Judge.
This is an appeal from an in rem proceeding brought under the Federal Food, Drug, and Cosmetic Act against a special dietary product “Nuclomin” claiming it is misbranded in violation of Section 403(a) of the Act, 21 U.S.C. § 343(a).1 Hunt Investment, Inc., owner of Nuclomin, intervened. Jurisdiction rests under 21 U.S.C. § 334. The district court upheld the government seizure and condemnation on the basis that several ingredients listed on the label were “either of no nutritional value per se or the quantities are so minute as not to enhance the nutritional value of the tablets.” The district court, the Honorable John K. Regan presiding, found that such label was false and misleading in that it could persuade a purchaser that the product possessed greater nutritional value than it actually did.
The basic issues on appeal include (1) whether the Food and Drug Administration (FDA) possessed the authority to prohibit the sale of a product that lists, as required by the regulations, completely safe ingredients that may be unnecessary or insignificant; (2) whether sufficient proof was presented to establish that the questioned ingredients were not needed or were included in inadequate amounts; and (3) whether the product label was in fact misleading. We affirm the trial court’s ruling.
THE FDA’S AUTHORITY
The government does not challenge the factual accuracy of the Nuclomin label; rather it claims that the label is misleading to the public because some of the ingredients are either not needed in human nutrition or are included in such insignificant amounts as to be valueless. Specifically, the government attacks the vitamin constituents choline, inositol and p-aminobenzoic acid, the mineral elements potassium, magnesium and calcium succinate, and the amino acids found in the yeast extract. It is undisputed that these ingredients are consumed daily by the public and are completely safe.
2. The Nuclomin label reads: