Intellectual Property in Plant Breeding
(1)
Max Planck Institute for Innovation and Competition, Munich, Germany
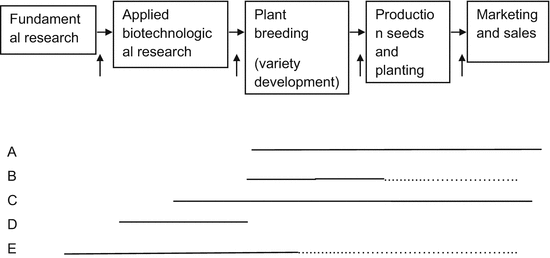
Intellectual property rights may be defined as rights granted by a state authority for certain products of intellectual effort and ingenuity. Intellectual effort and ingenuity applied in plant breeding has, however, not always been protected with property rights. Until the beginning of the twentieth century, the prevailing view was that IPRs were not suitable for plant protection: plants and other living organisms were considered as products of nature,1 rather than as fruits of human ingenuity. Later on, this view was mitigated by the advances of biotechnology, and different laws and court rulings at national level.2 The United States was the first country to provide for patents on plants with its 1930 Plant Patent Act. Afterward, in 1961, some European countries established the International Union on the Protection of New Varieties of Plants (UPOV), which provides for breeder’s rights, a special form of intellectual property.3 Several questions naturally arise: Why did countries change their view on plant protection? Are plants products of nature or fruits of human ingenuity? Is it beneficial for society to protect plants and their varieties? The historical, legal and economic aspects of the above issues have already been examined by several authors. This chapter contributes to previous literature by analyzing the legal and economic concerns on plant-related intellectual property protection in light of recent developments in plant breeding. To this purpose, the chapter is divided into two parts. The first part provides an overview of the main IPRs that have a direct influence in the plant breeding process, patents and breeder’s rights.4 A comparative view of these rights will allow us to better assess their role in plant breeding. The second part explains the rationale of IPRs, how it is applied in plant breeding and its relevance in the innovation process.
4.1 Legal Aspects of Intellectual Property in Plant Breeding
Intellectual property rights are enacted by national legislators and their form and scope may significantly vary across countries. However, countries that adhere to international IP agreements must comply with the minimum standards of protection provided for in these agreements. The Trade-Related Intellectual Property Rights Agreement (TRIPS) is the most comprehensive international agreement which sets minimum standards for the protection of intellectual property rights.5 This means that Member countries may provide for stronger IP protection but cannot elude the rules of TRIPS and enact weaker protection for IPRs. With regard to plant breeding, article 27 of TRIPS recognizes different forms of intellectual protection. It leaves the patenting of plants, biological processes for the production of plants and plant varieties to the discretion of WTO Members, but it requires Members to provide patent protection for microorganisms and non-biological and microbiological processes for the production of plants. In addition, its paragraph 3.b obliges countries to protect plant varieties ‘either by patents or by an effective sui generis system or by any combination thereof’.
This provision seems to recognize the diverse agricultural development and legislations of WTO members. Before the adoption of TRIPS, not all countries provided for property rights on plant varieties. Intellectual protection of plants was more idiosyncratic to developed countries. So, for example, the US and Australia protected innovations related to plant breeding through patents, while EU countries had chosen a combination of patents and breeders rights. Asian and African countries, on the other hand, had no specific legislation. After TRIPS, most of these countries signed the 1991 UPOV Convention in order to comply with the requirements of article 27.3.b.6 Others adopted a sui generis system designed to respond to their local socio-economic conditions by providing for rights for farmers.7
In order to further understand article 27 of TRIPS, it is important to distinguish between plants and plant varieties. From a botanical perspective, plants and their varieties are subdivisions of the Kingdom of Plantae.8 The taxonomic classification aims at grouping plants with similar morphological characteristics.9 Figure 4.1 aims to graphically represent this distinction with regard to the family of Rosaceae.
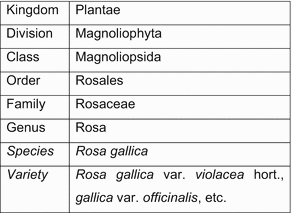

Fig. 4.1
Taxonomic classification of plants. For the classification of plants see the website of USDA Natural Resources Conservation Service. Available at https://plants.usda.gov/java/ClassificationServlet?source=display&classid=ROGA. Accessed 2 April 2015
Similar plants are placed in the same Genus. Individual plants within Genera are called ‘species’, while plants with slight differences from their ‘species’ are known as varieties. Varieties may be the result of changes in plant species that occur in nature through cross-pollination, mutation and adaptation or may be created with the help of human assistance.10 In the first case, varieties are highly heterogeneous and do not retain their distinctive characteristics when propagated. In the second case, human assistance prevalently aims at creating varieties that express the same distinguishing features through generations. The second type of plant variety is subject to breeder’s rights provided by UPOV whereas the first type of plant variety may be protected under sui generis regimes.11 In legal terms, a plant variety is ‘a plant grouping within a single botanical taxon of the lowest known rank, which grouping, irrespective of whether the conditions for the grant of a breeder’s right are fully met, can be defined by the expression of the characteristics resulting from a given genotype or combination of genotypes, distinguished from any other plant grouping by the expression of at least one of the said characteristics and considered as a unit with regard to its suitability for being propagated unchanged.’12 When bred varieties comply with these requirements, states may grant property rights on human ingenuity applied in the breeding process of new varieties. Plants, on the other hand, can be protected when their genome is altered in order to obtain modified plants. Changes in the genome of a plant are usually carried out by biotechnologists who insert gene constructs13 that confer new characteristics to the original plant. The modified (transgenic) plant cannot be qualified as a plant variety under breeder’s rights since it does not propagate unchanged.
This distinction between plants and plant varieties should be kept in mind when explaining different intellectual property rights. TRIPS gives countries the option to protect plants with patents, but obliges them to protect plant varieties with patents, an effective sui generis regime or a combination thereof. The provision of ‘an effective sui generis regime’ is controversial since TRIPS does define neither its form nor content.14 However, there seems to be general agreement that the breeder’s rights provided for by UPOV meet the requirements of article 27.3.b of the TRIPS Agreement.15 Moreover, many sui generis systems build upon the UPOV model. Thus, patents and breeder’s rights appear to be the most recurrent form of protection in plant breeding. The following paragraphs will provide the reader with a general understanding of these systems of intellectual protection. The focus of the analysis will be on the US and European legislations since most of the patents relevant for plant breeding are issued in these countries.
4.1.1 Patent Rights
As already explained in the introduction, TRIPS provides for the minimum requirements for patent protection at international level. Other regional agreements provide for similar requirements. In Europe, for example, the European Patent Convention (EPC)16 and the European Directive of 6 July 1998 on the legal protection of biotechnological inventions17 apply. These agreements and other national laws will be taken into consideration if relevant for the purpose of this thesis. Before starting with the analysis, it is necessary to clarify the concept of patent rights. Rights conferred with patents are negative rights. This indicates that the patent owner has only the legal faculty to prevent others from doing certain acts relating to the invention, but not the right to take actions on the patented invention. In practice, this means that the patentee of a transgenic plant has the right to prevent others from using the plant but he/she can commercialize the transgenic plant after complying with biosafety regulations.18
4.1.1.1 Object of Protection
Article 27 of TRIPS provides for patent rights for any inventions, whether products or processes, in all fields of technology. Thus, inventions are object of patent rights. But what is considered to be an ‘invention’? TRIPS does not offer a definition. Hence guidance will be found in national laws. With some exceptions,19 national laws do not explicitly define inventions, but rather what cannot be considered an invention.20 In this regard, it is worth noting that most countries do not consider discoveries to be inventions. The US Patent Act (PA), however, allows for both discoveries and inventions to be object of protection, provided that they are new and useful.21 Similarly, the US Plant Patent Act (PPA) grants patents for ‘Whoever invents or discovers and asexually reproduces any distinct and new variety of plant’.22 From these provisions, it can be inferred that ‘discovery’ and ‘invention’ have two different meanings. Whereas ‘discovery’ indicates the fact that something new is found, ‘invention’ implies the use of thought (human ingenuity) in the creation of something new.23 So, for example, when a breeder finds a new plant, he simply discovers it. Conventional wisdom would suggest that the breeder remains a discoverer even if she/he further breeds the plant and creates new varieties since the plant is a product of nature. UPOV, indeed, does not consider the breeder to be an inventor. US law, however, adopts another approach. The United States Patent and Trademark Office (USPTO) clarifies that ‘if one person discovers a new and distinct plant and asexually reproduces the plant, such person would be a sole inventor’.24 The term ‘inventor’ appears to be associated with the activity of discovery of a new plant. The simple discovery of a new plant is nevertheless not a sufficient condition to be object of patent protection. The PPA requires the discoverer to reproduce a new distinct variety of plant. It appears that the US concept of ‘invention’ refers to the efforts that the breeder puts in the breeding process.25 Thus, the borderline between discovery and invention for plants in the US seems to lie in the human work on discovered new plants. With regard to the US concept of ‘invention’ applied to biological matter, section 161 of the PA applies. According to this section, the invention or the discovery should concern ‘any new and useful process, machine, manufacture, or composition of matter, or any new and useful improvement thereof’. Biological matter can be considered under the terms ‘composition of matter’. In addition to the requirements for plant patentability, the discovery or invention of biological material forms object of patent protection only if it is useful.26
Further elucidations can be found in the 2012 Guidelines for Examination of the European Patent Office (EPO).27 The Guidelines identify ‘technicality’ as the main criteria for distinguishing between ‘discovery’ and ‘invention’. Accordingly, an invention is characterized by technical skills, technical features and application in a technical field. In these terms, a discovery may be patented if it applies to a technical solution of a technical problem.28 So, for instance, a gene with a particular function existing in nature is a mere discovery. But the isolation of this gene from its natural environment requires the application of technical skills, and therefore, may be considered as an invention.29 Article 3.2 of the EU directive clarifies that ‘Biological material which is isolated from its natural environment or produced by means of a technical process may be the subject of an invention even if it previously occurred in nature’. An ‘invention’, thus, is characterized by ‘technicality’ created by human ingenuity. The object of intellectual protection in this case is represented by the knowledge applied in the technical skills. It should be further noted that the terms ‘biological material’ used in article 3.2 of the directive, broaden the scope of patentability with regard to all living matter existing in nature. In order to advance reader’s knowledge on the subject, the following paragraphs will explain this concept of invention with regard to products and processes relevant for plant breeding.
4.1.1.1.1 Plants
Patents can protect both plant varieties and transgenic plants. Plant varieties can be explicitly protected through patents in Australia and in the US. By contrast, European laws do not allow for the patenting of plant varieties. Plant varieties, nonetheless, can be indirectly patented in Europe. According to the Board of Appeal of the EPO, ‘a claim wherein specific plant varieties are not individually claimed is not excluded from patentability (…)’.30 This means that a group of plants comprising more than one variety can constitute a valid patent claim, while a single plant variety does not. The rationale behind this choice lies in the differences of genetic constitution. As the Enlarged Board of Appeal (EBA) explained, ‘a plant defined by single recombinant DNA sequences is not an individual plant grouping to which an entire constitution can be attributed’, but an abstract and open definition embracing an indefinite number of individual entities defined by a part of its genotype or by a property bestowed on it by that part’.31 On the contrary, a plant variety as defined in UPOV and national European laws is characterized by its whole genome. According to the EBA, a plant defined by single recombinant DNA sequences is ‘neither limited nor even directed to a variety or varieties’. Here it is important to note that a patent may be issued on the gene sequence/s characterizing the modified plants, not on the variety. So, for example, a plant grouping containing a special disease resistant gene can be patented because it is characterized by the disease resistant element. Transgenic plants, which are characterized by such a special gene construct and have a technical application to a group of plants broader than the variety, can be patented in Europe as well as in the United States.
The US seems to offer the strongest form of protection for plants. Two types of patent rights, the 1930 PPA and section 101 of the Patent Act—offer protection respectively for asexually produced plants; asexually and sexually reproduced plants, part of plants including seeds and tissue cultures. Additionally, the 1970 Plant Variety Protection Act (PVPA) offers protection for seed-propagated plant varieties.32 Asexually propagated plants are those that are reproduced by cutting or grafting rather than by germinating seeds. Such plants are, for example, fruit trees and ornamental flowers.33 Varieties created by this type of breeding are identical to their parent plant. Not all asexually propagated plants are, however, protected by the PPA. This Act explicitly excludes tuber propagated plants such as the Irish potato and the Jerusalem artichoke. The US Manual of Patent Examining Procedure34 explains that such exclusion is justified on the basis that this group of plants is propagated by the same part of the plant that is sold as food.35 In any case, tuber propagated plants can be protected under the PVPA. Additionally, this Act provides for breeder’s rights for sexually reproduced varieties. Breeder’s rights are granted for any kind of seed-propagated variety, but they offer a weaker form of protection since they contain some exceptions.36 The so-called utility patents provided for by Section 101 of the PA provide for the most comprehensive form of protection. These patents may claim plants, seed, genes, methods for the production of plants, seeds, genes, etc. The US utility patents for plants are comparable to the standard patents usually granted in other countries.
4.1.1.1.1.1 Plants: Relevant Case Law for Patentability in Europe
The most important precedent in Europe is the ‘Novartis Case’, which has put the basis with regard to what can be patented.37 Patent claims, in this case, concerned transgenic plants transformed by recombinant DNA techniques to introduce pathogen resistance and also methods for preparing such plants. The original patent application made by Ciba Geigy (afterward Novartis, now Syngenta) was initially rejected by the EPO Technical Board of Appeal. Later on, the Enlarged Board of Appeal reversed the decision of the Technical Board of Appeal and held that patent claims were valid because they did not confine to a single plant variety. As clarified in the above paragraph, the Board held that a plant defined by a single recombinant DNA sequence neither expressly nor implicitly defines a single plant variety. Such a plant is not an individual plant grouping to which an entire constitution can be attributed. Therefore, a claim wherein specific plant varieties are not individually claimed is not excluded from patentability. Based on the interpretation of article 53 (b) of the EPC, the Board further explained that plant cells can also constitute relevant object matter because they are neither a plant nor a plant variety, but microorganisms. In addition, the EPO decision explained that a process for the production of plant varieties does not represent patentable subject matter.
4.1.1.1.1.2 Plants: Relevant Case Law for Patentability in the United States
In the US, three important precedents have shaped the protection of plants: the Diamonds v Chakrabarty,38 the Ex Parte Hibberd, 39 and the J.E.M. Agricultural Supply v Pioneer Hi-Bred International.40 The Diamonds v Chakrabarty was the first case to allow the patentability of living organisms by expressly stating that ‘a live human-made organism is patentable subject matter’. After this decision the relevant distinction for the patentability of biological matter is not between living and inanimate things but between products of nature, whether living or not, and human-made organisms. Subsequently, the Ex Parte Hibberd 41 established the right of plant breeders to patent sexually reproduced plants. This decision was upheld by the US Supreme Court in the J.E.M. Agricultural Supply v Pioneer Hi-Bred International. The Supreme Court explained that the lack of an explicit exclusion of plants in the provision of the PA that provides for utility patents provides no reason to view plant patentability as contrary to Congressional intent.
4.1.1.1.2 Plant Genes
A gene is a heredity unit located on a strand of DNA or RNA. Genes control the development of a trait or phenotype and can be self-replicated and transmitted to descendents.42 Plant genes, thus, are responsible for all plant characteristics. It is common knowledge that the existence of a gene is a product of nature. But can a gene form object of patent protection? Courts—both in the US and Europe—have affirmatively answered to this question by relying on the technical aspects of the concept of invention.43 So, for example, a DNA sequence or a partial DNA sequence qualifies for patent protection if it is isolated by means of a technical process and a specific function is identified.44 The mere location of a gene or gene sequence cannot be patented because it does not provide any technical information that may be usefully applied.45 Only the description of a specific use or function of the gene or gene sequence requires human ingenuity, and can therefore be deemed an invention in the USA and EU.
A specific function of a gene may be, for example, the production of a particular enzyme. But a single gene may have more than one function. Some of these functions may not be identified at the moment of the patent application. The issue raised in this regard concerns the patentability of other gene functions discovered after the first patent application. Do they fall within the scope of the first patent? Many authors agree that new gene functions cannot be exploited without the explicit consent of the first patent holder.46 This issue seems to have been solved in the case Monsanto Technology LLC vs. Cetefera BV and Others.47 The European Court of Justice explicitly stated that the EU directive prevents national laws from assuring to patentees rights beyond the purpose disclosed in the claims.48 In line with this reasoning, gene protection is limited only to the functions actually disclosed in the patent application.
With regard to subject matter eligibility, the recent case of Myriad Genetics offered important elucidations in the US.49 The US Supreme Court ruled in this case that ‘a naturally occurring DNA segment is a product of nature and not patent eligible merely because it has been isolated’.50 Additionally, the Court held that composite DNA (cDNA) is patent eligible because it does not naturally occur.51 cDNA represents strands of nucleotides that code for aminoacids (exons). Scientists may synthetically create such strands and exclude nucleotides which do not code for aminoacids (introns). According to the Court, the inventive aspect of cDNA lies in the efforts of the scientists to create something new that does not exist in nature.
Expressed sequence tags (ESTs) have also been subject of broad controversy with regard to patentability. ESTs are small pieces of DNA sequence (usually 200–500 nucleotides long) that are generated by sequencing either one or both ends of an expressed gene and are used in locating and mapping genes.52 Their function in plant breeding may be that of finding new plant genes, mapping the plant genome, and identifying coding regions in genomic sequences. The controversy underlying the patenting of ESTs is that they are sequences with an unknown function. The only recognized function is their use as a probe for screening libraries, identifying nucleotide sequences, and mapping their position within a genome. Although there is not yet explicit case law concerning the patentability of ESTs, it is generally accepted that ESTs are not patentable in Europe as long as their functions are not disclosed in order to fulfill the requirements for industrial application.53 The US jurisprudence, on the other hand, has made explicit that expressed sequence tags (ESTs) do not constitute a valid patent claim because of lack of specific and substantial utility and failure to comply with the disclosure requirement.54
4.1.1.1.3 Microorganisms
Article 27.3.b of TRIPS provides for patents on microorganisms. Microorganisms are usually defined as ‘living microscopic or submicroscopic organisms not observable by the naked eye.’ They include bacteria, fungi, archaea, protists.55 In contrast to this scientific definition, the EPO Guidelines opt for an open definition which encompasses ‘bacteria and other generally unicellular organisms (…), including plasmids and viruses and unicellular fungi (including yeasts), algae, protozoa and, moreover, human, animal and plant cells’.56 Although scientists have bespoken the limits of taxonomy and the problems in delineating species boundaries,57 EPO’s definition appears to challenge the scientific understanding of microorganisms. In order for microorganisms to form a species, they shall share some characteristics in common. Being an ‘unicellular’ microorganism might not be sufficient. Moreover, the classification of viruses as microorganisms is controversial. The controversy emerges from the fact that some scientists have erroneously relied on the infectious characteristics of viruses.58 A virus is more similar to cellular components rather than to microorganisms.59 However, it is worth noting that a more accurate definition would not influence virus patentability. In Europe as well as in the United States, patents can be issued on all biological material regardless of their living nature. Rule 26 of the EPC Implementing Regulations defines ‘biological material’ as ‘any material containing genetic information and capable of reproducing itself or being reproduced in a biological system’.60 Whenever these microbes are isolated from their natural environment or produced by means of a technical process, they may constitute an invention.
4.1.1.1.4 Non-biological and Microbiological Processes
TRIPS requires its Members to provide patent protection for processes, but it allows them to exclude essentially biological processes for the production of plants. This rule does not apply to non-biological and microbiological processes for the production of plants. On this point, TRIPS provisions seem to build upon European legislation. Therefore, an explanation of these technical terms can be found in the EPC and in the EU directive.61 Accordingly, ‘essentially biological’ refers to a process which consists entirely of natural phenomena such as crossing or selection, whereas the term ‘microbiological’ indicates a process that involves or performs upon or results in microbiological materials.
The definition of ‘essentially biological’ may open the way to different interpretations with respect to the natural criterion.62 The EPO offered an understanding of this issue by focusing on the degree of human intervention in Lubrizol/Hybrid Plants.63 The EBA judges clarified that the nature of human intervention in the process is decisive in qualifying a process as ‘essentially biological’. The simple fact that human intervention is required to assist the process is not relevant. What matters is whether human assistance has influenced the final result. The judges further explained that classical breeding methods are indisputably essentially biological despite the degree of human control. This is justified by the fact that breeding steps such as crossing and selection already occur in nature and are influenced by ‘complex, various, and non-predictable circumstances’.64 Therefore, natural breeding processes involve uncertainties. The role of the breeder in essentially biological processes is that of assisting what already occurs in nature. On the contrary, breeder’s role in non-essential biological processes is that of altering the natural breeding process by modifying the genome of the plant or by introducing new traits into its genome. On EBA’s understanding, this type of modification confers technicality to the process. It is human ingenuity that significantly changes natural breeding steps and controls the results of the process. Regardless of the biological steps the process involves, the utilization of at least one technical step which has an impact on the process or on the product obtained from it, determines its patentability. Marker-assisted selection (MAS) is an example of a patented process regardless of its use in breeding processes that consist on crossing and selection.65
This reasoning was reiterated by the Enlarged Board of Appeal in 2010, which further ruled that methods for conventional breeding of plants cannot be considered as a technical process and therefore cannot be covered by patents.66 Nevertheless, this ruling generated some legal uncertainty on the patentability of plants bred by methods of conventional breeding.67 The uncertainty seems to stem from the current rules which do not indicate whether plants obtained by essentially biological processes are patentable.68 This issue was solved by the Enlarged Board of Appeal in 2015, after nearly 10 years of review.69
4.1.1.2 Exclusions from Patentability
In some cases, biological material can be excluded from patentability even if it were object of an invention. Art. 27 of TRIPS provides for two exclusions. A general exclusion for reasons of ordre public or morality is found in its paragraph 2, whereas a specific exclusion for plants is contemplated in paragraph 3.b. An understanding of these exclusions will be provided below.
4.1.1.2.1 Exclusions for Ordre Public or Morality
Members may exclude from patentability inventions, the prevention within their territory of the commercial exploitation of which is necessary to protect ordre public or morality, including to protect human, animal or plant life or health or to avoid serious prejudice to the environment, provided that such exclusion is not made merely because the exploitation is prohibited by their law. (emphasis added).
It is important to note that this paragraph does not, sic et simpliciter, allow for exclusions on grounds of ordre public or morality.70 This can be deduced from the term ‘necessary’ as well as from the need to prohibit the commercialization of the invention. Based on WTO dispute settlement panel decisions, a measure is ‘necessary’ only when there is no alternative measure available,71 whereas the need to prohibit the commercialization of the invention is decided by national laws. This provision seems to reflect article 53.a of the EPC. The US legislation does not take into consideration any constrains on patents based on ordre public or morality.
4.1.1.2.2 Exclusion of Plants
The third paragraph of article 27 allows for the exclusion of plants and essentially biological processes for the production of plants from patentable subject matter. Since essentially biological processes were discussed in the above paragraphs, the exclusion of plants will be here object of attention. Under TRIPS, states have the option to exclude plants form patentability, but they are obliged to provide for protection on plant varieties and for patents on microorganisms. This has two consequences. Firstly, even if states exclude a plant from patentability, protection for its varieties should be still available. Secondly, if a patented gene construct is inserted into a plant, the effects of patent protection will extend to the plant itself. This second consequence renders void the exclusion of plants from patentability. Therefore, countries that exclude patents on plants should consider excluding parts of plants as well.72
4.1.1.3 Requirements for Protection
Countries must grant patents on the above described plant-related innovations subject to three conditions laid down in TRIPS: novelty, inventive step, and industrial application. Since TRIPS does not offer a definition of these requirements, their interpretation and application may differ among Member countries.
4.1.1.3.1 Novelty
The compliance with ‘novelty’ demands countries to patent only those inventions that are original and new with respect to the state of the art. The definition of the state of the art entails the disclosure of the invention by any means to the public.73 Prior to the coming into force of the America Invents Acts (AIA) on 16 March 2013, the US adopted a different understanding of novelty which allowed patenting plant-related material known in other countries, but not divulged in written form. This generated several controversies. The US patent on basmati rice granted to the RiceTec company is a well-known case for raising biopiracy concerns on the Indian rice variety.
4.1.1.3.2 Inventive Step/Non-obviousness
Once the novelty requirement is satisfied, the invention should have an inventive step or should not be obvious to a person skilled in the art. The first criterion is applied in Europe, whereas the second in the US. Despite similarities in assessing these criteria, they have different meanings. The ‘inventive step’ requirement denotes an intellectual process to develop the invention. ‘Non-obviousness’, on the other hand, may exclude intellectual effort when the subject matter is simply found.74 Indeed, the evaluation of these criteria appears to be different under EPO and USPTO practice. The EPO evaluates the inventive step requirement on the basis of the ‘problem-solution’ approach. This means that the solution claimed in the invention shall not be evident to the person skilled in the art.75 For the USPTO, the decision on obviousness considers ‘whether the improvement is more than the predictable use of prior art elements according to their established functions’.76 Both Offices, however, determine the criteria with regard to common knowledge of those skilled in the art. If the claimed invention is evident to those skilled in the art, it means that the invention lacks ingenuity, and therefore, fails to introduce a novel step in the state of the art.
4.1.1.3.3 Industrial Applicability/Utility
According to article 57 of the European Patent Convention, ‘industrial application’ denotes that the object of invention can be made or used in any kind of industry, including agriculture. Besides agriculture, plants and plant-related innovations may find application in other industries such as the pharmaceutical, biofuel, chemical, and the cosmetic sector. Section 101 of the US Patent Act, on the other hand, requires patents to be useful. ‘Utility’ slightly differs from ‘industrial application’ since a useful invention might not always have an industrial application.77 The key to interpreting ‘utility’ lies in enabling one skilled in the art to ‘use the claimed discovery in a manner which provides benefit to the public’.78 US Courts have clarified that the benefits should be specific and well-defined. Translated into practice, this means that the invention should disclose specific uses instead of broad and general claims. Additionally, the ‘utility’ shall be substantial in the sense that the benefit has to be significant and presently available to the public.79 In other terms, the invention should have a ‘real world’ use and should not require further research to determine its utility.
4.1.1.3.4 Sufficiency of Disclosure
In addition to the above mentioned criteria, article 29 of the TRIPS Agreement establishes that a ‘patent application shall disclose the invention in a manner sufficiently clear and complete for it to be carried out by a person skilled in the art’.80 This provision ensures that the invention depends on human ingenuity instead of random coincidence.81 Only those inventions that rely on systematic scientific research enable others skilled in the art to further reproduce for the benefit of the public. But the reproducibility of the invention presents a challenge for biotechnological inventions. It is very difficult to describe biological matter in such a way that another skilled in the art recreates the same organism with an identical genetic code. US law loosens the disclosure requirement for asexually propagated plants. Based on section 162 of the PPA, a clear description is not necessary for the grant of a plant patent. What is important for other breeders to reproduce the plant is the description of the plant ‘as complete as is reasonably possible.’ In other words, plant patent claims combined with knowledge in the prior art, replace the disclosure requirements provided for other biological material. Protection of plant material required under the PPA is also not subject to the deposit rules on biological matter. However, deposit of plant material is obligatory for utility patents.82 This facilitates access and reproduction of protected plant material.
4.1.1.4 Scope of Protection
Article 28 of TRIPS specifies the minimum patent rights that countries should provide for products and processes.83 Paragraph 1 of this article confers exclusive rights with regard to the acts of making, using, offering for sale, selling or importing for any of these purposes the patented product. From the wording of this provision, patent protection seems to be confined to the object of invention. However, the matter is different with regard to biological material. Patentee’s rights cover products that contain or derive from patented material. A few elucidations can be found in articles 8.1 and 9 of the EU directive. According to article 8, if a patent is granted on biological material possessing specific characteristics, the protection extends to all biological material derived from the patented biological material provided that the derived material possesses the same characteristics. Article 9 further clarifies that patent protection embraces all material where patented genetic information is contained and performs its function.
These provisions have in common the characteristic of extending patent protection to biological material that maintains its inventive features and function. Thus, inventive features and function represent the limit for exercising patent rights. Since biological matter might self-replicate indefinitely, concerns arise with regard to the principle of exhaustion of rights. According to this principle, the patent owner cannot control the use of the product after its first sale. A controversy on this point has been raised in Bowman v Monsanto. 84 The case concerns a US farmer who acquired commodity seed from a grain elevator containing Monsanto patented seeds. After planting and harvesting Monsanto seeds, Bowman replanted some of them for eight consecutive years. The main question that this case poses is whether the replanting of Monsanto seeds constitutes ‘use’ of a patented product, and thus infringes the patent, or the authorized sale of seeds justifies the application of the patent exhaustion doctrine and allows Bowman to legitimately replant patented seeds. The Court ruled that the patent exhaustion doctrine allows Bowman to plant patented seeds in one season, and subsequently consume the resulting crop or sell it as a commodity, but not to reproduce them through planting and harvesting without patentee’s consent. This reasoning was based on the fact that the patent holder had ‘received his reward’ only for the sold seeds, not for subsequent replanting.85 Conversely, Bowman argued that by replanting harvested seed, he was ‘merely using them in the normal way farmers do’. In the Court’s understanding, replanting harvested seed is equivalent to ‘making a new product’. It could be argued that the genetic constitution of the replanted seeds is different from the patented Monsanto’s Roundup Ready soybeans. Therefore, Bowman has not ‘copied’ Monsanto’s soybean seeds. But it is also true that the harvested soybeans contain the genetic modified Roundup Ready seeds. Without doubt, the self-replicating nature of patented material poses serious difficulties to patent exhaustion. Unlike other commodities, the scope of patent protection on biological products appears to be overbroad. Besides the principle of patent rights’ exhaustion, another important issue that the broad scope of protection for biological material raises is the indirect patentability of plants when they contain or consist of patented material. As a result, countries that exclude plants form patentable subject matter are forced to provide an indirect protection.
Contrary to patent rights on products, the rights on processes appear less stringent. Paragraph 2 of article 28 confers exclusive rights with respect to the utilization of the process as well as to the product directly obtained by that process. Although the rights on the obtained product are the same as those of the first paragraph, here it is made explicit that these rights concern ‘at least’ the product directly obtained by the patented process. Nevertheless, countries have chosen to broaden the scope of patent protection. Article 8.2 of the European directive, for example, extends the scope of protection to all biological material derived from the directly obtained product.
Not all processes, however, aim at creating a product. It should be differentiated between working and production processes.86 Working processes do not result in a product (plant-related material), whereas production processes aim at creating a product. Given that working processes do not lead to product creation, the scope of patent protection does not extend to the product. If plant breeding were classified as a working process, there would be no extension of patent protection on breeding products. But is plant breeding a working process? Although some of the plant breeding steps are working processes—i.e. selection of parents and selection of offspring with desired traits—other steps, such as crossing of selected parents to produce offspring and propagation of offspring clearly result in products and are, therefore, production processes. Furthermore, being the objective of plant breeding that of the creation of new plants, it seems reasonable to classify it as a production process.
Process protection poses a few problems with regard to the product ‘directly’ obtained from the process. Processes may result in several products, but are all of them ‘directly’ obtained? It has been suggested that the term ‘directly’ implies a direct relationship between the patented process and the product. In other words, patent claims should cover all material and important steps that lead to the creation of the product.87
4.1.1.5 Exceptions and Restrictions to Patent Rights88
TRIPS provides for exceptions to patent rights in its article 30. It does not list acts that may be excepted but gives countries the option to provide limited exceptions to the rights conferred by a patent subject to the following conditions: (1) the exceptions should not unreasonably conflict with a normal exploitation of the patent; (2) should not unreasonably prejudice the legitimate interests of the patent owner; (3) they should take account of the legitimate interests of third parties. This vague formulation does not permit to draw immediate conclusions with regard to the type of activities that may be exempted from patent law. Therefore, it is useful to look at national legislations to assess the practice of countries in this regard.
US law lacks specific rules on patent exceptions,89 whereas EU countries have adopted exceptions that reflect the provisions of articles 10 and 11 of the EU directive. Article 10 allows reproducing patented biological material when the multiplication or propagation necessarily results from the application for which the biological material was marketed. But subsequent propagation or multiplication of the reproduced material is not permitted. This provision seems to confirm the basic exceptions which existed prior to the EU directive on biotechnological innovations. These basic exceptions concern the use of the patented products for private and research purposes. Thus, patent rights do not extend to private individuals reproducing the patented subject matter for their own use and to those parties that use the patented innovation in scientific research activities. Some countries additionally allow for the exception of research activities that aim at the development of a commercial product or process.90 However, final commercialization is not exempted. Additionally, paragraph 1 of article 11 provides for a farmer’s exception by permitting farmers to use the harvest deriving from patented material.91 This exception is unique to the EU directive since no other patent law provides for patent exceptions in favor of farmers.
A recent exception to patent law is the so-called breeder’s exception or breeding exception. The breeding exception allows for the free use of patented biological material limited to the breeding or discovery and development of new varieties. Four European countries, France, Germany, the Netherlands and, Switzerland have already adopted this type of exception into their patent laws. A provision on the breeding exception has also been incorporated in article 27.c of the recent Agreement on a Unified Patent Court (AUPC). This agreement has not yet entered into force,92 thus, it remains to be seen whether EU countries will implement such provision into their national patent law.
Besides exceptions, the grant of compulsory licenses by national governments represents another restriction to patent rights. To regulate the compulsory licensing of patented products and processes, TRIPS offers a complex set of rules in its article 31. Compulsory licenses may reveal to be particularly relevant in plant breeding because of its dependence on patented products or processes. This peculiarity of plant breeding innovation has especially been taken into consideration by the EU directive, which provides for a compulsory license for non-exclusive use of patented inventions as well as protected varieties in its article 12. In both cases, the license is subject to the payment of an appropriate royalty. It should be, however, noted that the specific conditions required by article 12.3 of the EU directive are difficult to satisfy. Article 12.3 (a) obliges applicants to demonstrate that they have been denied authorization to use the protected material. As it will be explained further in this chapter, patent holders often do not reply to licensing requests. Thus, it is difficult to provide proof of an explicit denial. It may be argued that breeders might adopt the same behavior with patent holders asking for a license on a plant variety. However, the need of patent holders to obtain a license from a plant breeder might appear as a remote possibility. It will be clarified in the next sections that plant breeder’s rights confer protection on the variety as a whole and at the same time allow other innovators to use the variety.93 This means that patent holders do not need to obtain a license for using the variety. If they have an interest in accessing patented genetic material of the protected variety, they can use advanced biotechnological methods such as MAS or reverse breeding. Therefore, a license on a plant variety might be unnecessary.94 Article 12.3 (b) of the EU directive further requires the applicant to demonstrate that the ‘plant variety or the invention constitutes significant technical progress of considerable economic interest compared with the invention claimed in the patent or the protected plant variety’. It is not easy to show the significance of the technical progress and the economic interest embodied in the plant variety or invention if a product has not yet been developed.95 Only the potential to bring technical progress and economic benefits might be illustrated at this stage. Applicants should rely on the vagueness of terms like ‘significant’ and ‘considerable’ to advance their interests.
4.1.1.6 Period of Protection
Article 33 of the TRIPS Agreements sets a minimum term of 20 years from the filing of the patent application.
4.1.1.7 Critical Assessment
Patent rights on biological material raise a plethora of concerns with respect to economic, legal, and ethical aspects.96 Since economic aspects will be explained in the second part of this chapter, this section focuses only on the legal and ethical issues of patenting relevant for plant breeding.
4.1.1.7.1 Legal Concerns
One of the main purposes of legal rules is that of reducing uncertainties. The current rules on patentability seem to fail on this point. TRIPS creates a first confusion when it allows countries to exclude plants from patentability but it requires them to provide for patents on microorganisms. Several authors have argued that this results in a nullification of the option to exclude plants from patentability.97 Hence it is not clear how countries can successfully implement this option on plant patentability. A similar concern can be raised for plant varieties in European laws. Although the EPC excludes patents on plant varieties, it is legally possible to obtain a de facto protection on plant varieties through patents on plant-related material. This can be attained in one of the following ways:
1.
the technical feasibility of the patented invention is not confined to a specific variety;
2.
a patented process claims a non-essentially biological process for the production of plants;
3.
when a patented gene construct is introduced into the variety.
Another vagueness of EU law is related to the scope of patent rights on biological material. As explained above, the patentee can exercise his rights on biological material as long as the material performs its function. Since patentee’s rights extend to any other material where the invention is incorporated, patent rights cover material which may not fall under patentable subject matter. While specific rules limit the extension of such rights to human body, there are no similar provisions for plant breeding. Given that plant breeding is our source of food supply, patent rights on plant biological material have a direct impact on our daily lives. A landmark case, Monsanto v Cargill can better clarify the issue at hand.98 Cargill imported into the UK soybean meal from Argentina, which was made from Monsanto’s genetically modified soybeans. In specific, patent claims concerned DNA sequences that allow soybeans to express an enzyme that confers resistance to the herbicide glyphosate (RoundUp Ready®). Given that the Monsanto patent was disallowed in Argentina, Monsanto claimed infringement of its patent after importation into the UK. Based on article 9 of the EU directive, the dispute was about whether the genetic information embedded in the plant still performs its function when grain and flour of the patented soybean is traded. In the UK, judges expressly rejected the argument that the soybean meal was a direct transformation of the patented DNA molecule and concluded that the DNA in the soymeal did not perform the function for which it had been patented. Monsanto tried to prove infringement of his patents also in Spain and in the Netherlands, where soybean meal was imported. Spanish judges arrived at the same conclusion of UK judges, whereas the District Court of The Hague submitted a number of questions to the European Court of Justice (ECJ) with regard to the applicability of article 9 of the EU directive in the present case. The ECJ clarified that article 9 is not applicable ‘when the genetic information has ceased to perform the function it performed in the initial material from which the material in question is derived’.99 Although different courts excluded the extension of patent rights to processed meal, others might take opposite decisions. This is mainly because there are no rules that limit the scope of patent protection in plant breeding. Therefore, it would be desirable that states adopt specific limits for patents that affect our daily food.
Other uncertainties of patent law regard patent quality. A patent is deemed to be of quality when it protects innovations that are new, involve an inventive step, have an industrial utility, and that have valid, well-defined, and clear claims. When patents do not satisfy these criteria, they can be opposed and invalidated. Obviously, patent litigation involves considerable social costs. This provides an intimately link between legal uncertainties on patent quality and costs on the society. Therefore, a critical analysis of patentability requirements is extremely important in order to reward only true inventions that bring social benefits.100 The way in which patentability criteria are being evaluated by patent offices has been object of growing worries in recent years.101 The EPO itself admits the risk of misinterpreting patent requirements as a result of increasing patent filings, which slows down the examination procedures.102 US courts on the other hand, have started to adopt a stricter interpretation of patentability requirements in some cases. An imminent example was the rejection of patents on expressed sequence tags because of insufficient proof of utility.103 With regard to the inventive step, biotechnological advancements pose new challenges to patentability criteria. New discoveries make existing technologies obsolete. For example, gene sequencing hardly involves an inventive step nowadays. This has led some authors to require a redimensioning of patent requirements.104 The US jurisprudence has already started to go in this direction by signaling in RE Kubin that significant advances in technology raise the level of what is to be considered obvious to one of ordinary skill in the art.105 The ‘novelty’ requirement has also come under the scrutiny of US judges which have invalidated patent claims on unknown gene properties that already exist in plants or existing methods for preparing food containing such properties. Here the invention would concern the simple discovery of useful plant characteristic. In this respect, the US judges have clearly stated that the mere description of unexpected beneficial results of a known process is an invalid claim because of lack of novelty.106 This decision, however, has not prevented companies from patenting other similar claims. Monsanto, for example, has obtained a patent covering a virus-resistant melon plant, its parts, fruits, and seeds, modified by introducing a publicly available gene first found in melon plants in India.107 The patent has already been opposed by both biotech firms and civil society organizations.
The formulation of patent claims further complicates the debate on patent quality. A study has pointed out that patent claims on breeding technologies are too vague. They describe the process of the technique without indicating a specific trait or plant to be obtained.108 For example, it was found that a patent concerning the breeding technique of RNA-dependent DNA methylation (RdDM) claims no specific plant species. It simply claims that gene silencing can be directed towards harmful genes for the plant or unwanted traits like over-ripeness. Vagueness of claims often leads to broadness of patent’s scope. For example, by non specifying the relevant plant species, the above patented method can be enforced on all plant species. Furthermore, patentees tend to extend the patent claims on a wide range of products and processes without knowing in advance if the innovation can be applied to each of these claims. The following example can illustrate this point:
The methods and means described herein are believed to be suitable for all plant cells and plants, both dicotyledonous and monocotyledonous plant cells and plants including but not limited to cotton, Brassica vegetables, oilseed rape, wheat, maize or corn, barley, alfalfa, peanuts, sunflowers, rice, oats, sugarcane, soybean, turf grasses, barley, rye, sorghum, sugar cane, vegetables (including chicory, lettuce, tomato, zucchini, bell pepper, eggplant, cucumber, melon, onion, leek), tobacco, potato, sugar beet, papaya, pineapple, mango, Arabidopsis thaliana, but also plants used in horticulture, floriculture or forestry (poplar, fir, eucalyptus etc.) (a patent registered by Bayer BioScience NV, EP2449108 A1, emphasis added).
Words such as ‘are believed to be suitable’ do not indicate certainty on the application of the patented methods on the claimed plants, but only a mere hypothesis that has not yet been verified. In addition, the above example shows a broadly formulated claim. Broad claims grant market power as well as keep open the possibility to strengthen market power whenever the claimed uses are put into practice in the future.
It goes without saying that all these concerns do not offer legal certainty. In such a situation, patent law may impede plant breeding innovations instead of furthering them. Therefore, countries should emanate clearer rules on the patentability of biological material.
4.1.1.7.2 Moral and Ethical Concerns
Distinction should be made between ‘ethics’ and ‘morality’. These terms are very often used interchangeably but they have different meanings. Whereas ‘morality’ is used to differentiate between ‘good’ and ‘bad’ based on the principles of a particular group or individual, ethics is ‘a set of concepts and principles that guide us in determining what behavior helps or harms sentient creatures’.109 Thus ethics does not dictate moral rules but it serves as a means to determine ‘morality’. In terms of the issue at hand, both morals and ethics are important. The sources of morals are various. Morals based on religious beliefs dictate that life is sacred. Hence any attempt to provide for property rights on life forms may be deemed immoral. What is more, God is the only creator of any form of life, thereby there can be no invention of any biological material.110 The morals based on law, on the other hand, accept patenting of biological matter when humans modify nature to realize social goals. The controversy over the patentability of life forms is, however, not confined to law and religion. A critical observation of natural phenomena finds that every kind of life form stems from an interaction of precise laws and energy cycles existing in nature. Human beings can control and direct natural phenomena, can find new scientific laws, but cannot create them from scratch. Hence humans can ‘innovate’ but not ‘invent’. In this view, ‘invention’ is interpreted in function of nature supremacy. Some, indeed, accept that nature reigns over mankind and refrain from any action that might disrespect her. Others, however, believe in the dominant abilities of men and reward their creations for improving nature. When the reward goes to innovators who do not compensate the efforts of traditional farming, the reward is often seen as immoral. This phenomenon is known with the term ‘bio-piracy’ and generates great societal worries. All these views are important given that societal wellbeing is determined by the welfare of its individuals. But the prevalence of one view over the other may lead to social discontent.111 This is currently the case with respect to the patentability of biological material.
Turning to the ethical aspects of patents on biological matter, it is sustained here that universally accepted values on human life should guide what ‘behavior helps or harms sentient creatures’. Universally accepted values are found in UN documents such as the Universal Declaration of Human Rights (UDHR) and the International Covenant on Economic, Social and Cultural Rights (ICESCR). Article 25 of the UDHR and article 11 of the ICESCR are relevant in the context of plant breeding. Both these articles state that food is a component of an adequate standard of life. Article 11, in particular, recognizes the right to food and to be free from hunger. Food and patents are nowadays closely linked. When patents are issued on plants, their varieties or plant materials, the final price of the products reaching the market is higher. This might not necessarily affect the budget of consumers since markets offer multiple choices. Nevertheless, patented food affects the buying power of consumers in poor countries. In this case, patents would harm the interests of poor people.112 In terms of ethics, the universal value of ensuring access to food and eliminating hunger indicate that patenting activity harms some communities. Therefore, it appears that patenting is not ethical with respect to the right to food. Patenting, however, brings substantial monetary benefits to firms and to the countries where they do business. A growing economy undoubtedly benefits people living in these countries. In rich countries, patents may support food supply since theoretically they bring about innovations. But this is not sufficient to conclude that patents are ethical. A critical approach would recall universally accepted principles. In this respect, article 1 of the UDHR appears as the most important. It recommends that all human beings ‘should act towards one another in a spirit of brotherhood’. The concept of ‘spirit of brotherhood’ impels the rich to alleviate the suffering of the poor. Such a broad approach to societal problems is the premise for sustainable development. In this context, benefits surmount the monetary costs that some might endure. Therefore, it appears that eliminating patents is ethical whenever the right to food comes into play. It should be noted, however, that in absence of patents, biological inventions can be protected by other rights.
4.1.2 Breeder’s Rights
Breeder’s rights are an intellectual protection system specifically designed for the breeding of new varieties of plants. They confer to the holder the right to exclude others from a number of activities related to the protected variety. The first efforts to provide varietal protection date back to the early decades of the twentieth century. Following the Czechoslovak and French laws in 1921 and 1922—other countries like Austria, the Netherlands and Germany—introduced plant breeder’s rights respectively in 1938, 1941 and 1953.113 These national laws were afterward harmonized by the UPOV Convention in 1961.114 After several amendments in 1972, 1978, and 1991, the 1991 version is in force in most countries.115 The US and the EU has also ratified the latest version. Therefore, the following paragraphs will explain the characteristics of plant variety protection based on the 1991 UPOV.
4.1.2.1 Object of Protection
Contrary to patents, breeder’s rights do not protect an invention but the improvement of existing varieties. As a matter of fact, there is no real invention in the field of plant breeding.116 Plant varieties are products of breeders as well as of nature. When breeders create a variety, there is no guarantee that other breeders will produce the same variety. The specific outcomes of each crossing are subject to random variation and thus neither predictable nor necessarily reproducible. However, breeders can intervene to ‘modify’ nature for their own purposes and create varieties with desirable traits. These modifications confer to the plant variety different characteristics from the original plant species. It is, thus, the betterment of the original characteristics of plant species that is deemed to be worthy of intellectual protection.
Innovation may consist in ‘breeding’ a new variety or in ‘discovery and development’ of a new variety. The simple discovery of a new variety cannot be qualified for UPOV protection since the discovered variety is a mere product of nature. If the breeder further improves the discovered variety in order to produce a stable and distinct variety, the outcome can be qualified for breeder’s rights protection. For instance, a red flowering variety may develop from a plant species that is known to bloom pink. The red flowering variety is not subject to breeder’s rights unless breeders develop a stable and uniform line of the distinct red flowering variety.
4.1.2.2 Requirements for Protection
Assuming that a plant variety falls within a protected genera or species, it is eligible for protection only if it is new, distinct, uniform, and stable.117 These conditions are explained below.
4.1.2.2.1 Novelty
Given the particular nature of innovation in plant breeding, the characteristic of novelty does not refer to varieties not existing in nature, but to varieties not having been exploited in the market for a certain period before the filing date of the application for the grant of breeder’s rights. In the country where the application is submitted, the term required is 1 year, whereas in other countries it provides for 4 years for plant varieties and 6 years for trees and vines.