Doha Declaration and Beyond
9
Doha Declaration and Beyond
THE DECLARATION ON the TRIPS Agreement and Public Health,1 adopted at the WTO Doha Ministerial Conference in 2001, responded to growing concerns about the implications of the TRIPS Agreement for access to medicines. It declares that the TRIPS Agreement does not and should not prevent members from taking measures to protect public health. The Declaration refers to ‘flexibilities’ that this Agreement provides and ensures that WTO Members may resort to such flexibilities, including compulsory licensing, to cope with public health problems.
This chapter describes the content of the Declaration and different views about the nature and scope of the flexibilities that the Declaration refers to. The relationship between the TRIPS Agreement and the Doha Declaration is also explored. Albeit with extremely limited data, this chapter looks into the circumstances in which certain compulsory licences were issued during the ten years after the adoption of the Declaration. The chapter examines administrative and court IP policies on compulsory licensing in some of the rapidly industrialising, middle-income developing countries, with a view to exploring how public interest could be intermingled with industrial policy considerations.
I THE ADOPTION OF THE DOHA DECLARATION
A Doha Development Agenda (DDA)
At the Fourth Ministerial Conference which took place from 9 to 11 November 2001 in Doha, Qatar, WTO Members agreed to launch the ninth multilateral negotiations to be carried out since 1948, defining its work programme in the Ministerial declaration of 14 November 2001. The main areas of negotiations were agriculture, market access for industrial goods, services, trade facilitation, WTO rules (ie, trade remedies, fish subsidies, and regional trade agreements) and development.2 The Conference also adopted the Decision on Implementation-Related Issues and Concerns,3 which identifies ways to resolve the difficulties and resource constraints faced by developing countries in implementing the current WTO Agreements. Those implementation issues on which no clarification was made were not included in this Decision, but were included among the items to be negotiated,4 and the approximately 20 remaining issues were to be addressed as a matter of priority by the relevant WTO bodies, to report to the Trade Negotiations Committee (TNC) for discussion on possible inclusion as issues to be negotiated.5 Those issues which the TNC decided to give a negotiating mandate would become part of the work programme. This entire package was called the Doha Development Agenda (DDA).
Concerning the TRIPS Agreement, the following declarations were made in the Ministerial Declaration:
- the importance of implementing and interpreting the TRIPS Agreement in a manner supportive of public health, by promoting both access to existing medicines and research and development into new medicines;6
- the establishment of a multilateral system of notification and registration of geographical indications for wines and spirits;7
- the examination as implementation issues of the built-in agenda, ie, the review of Article 27.3(b), the relationship between the TRIPS Agreement and the Convention on Biological Diversity (CBD),8 the protection of traditional knowledge and folklore, and other relevant new developments raised by Members pursuant to Article 71.1.
In undertaking 3 above, the Decision notes that, ‘the TRIPS Council shall be guided by the objectives and principles set out in Articles 7 and 8 of the TRIPS Agreement and shall take fully into account the development dimension’.
B The Doha Declaration: What It Says
The Doha Declaration on the TRIPS Agreement and Public Health (Doha Declaration) was adopted at the WTO Ministerial Conference on 14 November 2001, outside of the Work Programme. The Ministers recognised ‘the gravity of the public health problems afflicting many developing and least-developed countries, especially those resulting from HIV/AIDS, tuberculosis, malaria and other epidemics’ (paragraph 1) and stressed ‘the need for the WTO TRIPS Agreement to be part of the wider national and international action to address these problems’ (paragraph 2). It expressed the concern that patents have an impact on prices, while recognising that IP protection is important for the development of new medicines (paragraph 3).
The main message of the Doha Declaration is in the first sentence of paragraph 4. The Ministers agree that ‘the TRIPS Agreement does not and should not prevent Members from taking measures to protect public health’. At the same time, the Ministers, ‘reiterating [their] commitment to the TRIPS Agreement, affirm that the Agreement can and should be interpreted and implemented in a manner supportive of WTO Members’ right to protect public health and, in particular, to promote access to medicines for all’ (second sentence of paragraph 4).
The third sentence of paragraph 4 states: ‘In this connection, we reaffirm the right of WTO Members to use, to the full, the provisions in the TRIPS Agreement, which provide flexibility for this purpose.’ Paragraph 5 reiterates the phrase ‘while maintaining our commitments in the TRIPS Agreement’, and recognises that the above flexibilities include:
a) in applying the customary rules of interpretation of public international law, each provision of the TRIPS Agreement shall be read in the light of the object and purpose of the Agreement as expressed, in particular, in its objectives and principles;
b) each Member has the right to grant compulsory licences and the freedom to determine the grounds upon which such licences are granted;
c) each Member has the right to determine what constitutes a national emergency or other circumstances of extreme urgency, it being understood that public health crises, including those relating to HIV/AIDS, tuberculosis, malaria and other epidemics, can represent a national emergency or other circumstances of extreme urgency; and,
d) the provisions in the TRIPS Agreement that are relevant to the exhaustion of intellectual property rights leave each Member free to establish its own regime for such exhaustion without challenge, subject to the most-favoured nation and national treatment provisions of Articles 3 and 4.
Paragraph 6 refers to the difficulties that WTO Members may face, even if a compulsory licence is issued, if they have insufficient or no manufacturing capacity in the pharmaceutical sector. The Ministers instructed ‘the Council for TRIPS to find an expeditious solution to this problem and to report to the General Council before the end of 2002.’
Paragraph 7 reaffirms the provision concerning the transfer of technology to LDCs under Article 66.2 of the TRIPS Agreement and extends the transition periods for LDCs regarding pharmaceutical products in implementing and applying Sections 5 (patents) and 7 (undisclosed data) of Part II of the TRIPS Agreement until 1 January 2016, renewable upon requests in accordance with Article 66.1.
C Legal Status of the Declaration
All commentators agree that the following decisions taken by the TRIPS Council in pursuance of the Doha Declaration on Public Health are legally binding. The TRIPS Council, by Decision IP/C/25 of 27 June 20029, decided that least-developed country Members will not be obliged, with respect to pharmaceutical products, to implement or apply Sections 5 (patents) and 7 of Part II (undisclosed data) of the TRIPS Agreement or to enforce rights provided for under these Sections until 1 January 2016. This decision was taken following the ‘instructions’ of the Ministerial Conference to the Council for TRIPS contained in paragraph 7 of the Doha Declaration on Public Health which the Council considered as constituting a duly motivated request by the least-developed country Members for an extension of the period under Article 66.1 of the TRIPS Agreement. The TRIPS Council Decision L/540, adopted on 30 August 2003 pursuant to paragraph 610 of the Declaration, establishes the mechanisms for compulsory export licensing for countries with insufficient or no capacity to manufacture medicines (on paragraph 6 system, see below p 314).11
All commentators consider the Declaration to be an important political and moral declaration with practical implications. Many commentators hold that the Declaration does not change Members’ rights and obligations under the TRIPS Agreement, although it has become politically more difficult to exercise these rights.12 Correa, on the other hand, asserts that the Declaration is a ‘Ministerial decision with legal effects on the Members and on the WTO bodies, particularly the Dispute Settlement Body and the Council for TRIPS.’ According to Correa, the Declaration implies that ‘public health-related patents may be treated differently from other patents’.13
The Declaration does not indicate that it is an ‘authoritative interpretation’, as defined in Article IX.214 of the Agreement Establishing the WTO, and its adoption did not follow the procedures foreseen in the above Article. Its draft was negotiated in the TRIPS Council and was sent to the General Council.15 Jackson observes that the Doha Declaration gives the impression that it was adopted only by those Ministers who participated in the meeting concerned, and not by the Ministerial Conference.16 The Declaration was adopted at the Doha Ministerial Conference but the Ministerial Conference did not take the three-quarters majority decision required to adopt an ‘authoritative interpretation’. If the Declaration is not an ‘authoritative interpretation’ that requires a procedure less onerous than those for treaty amendments delineated in Article X of the WTO Agreement, it is, a fortiori, not an amendment of the TRIPS Agreement.
Correa considers this Declaration to have the same effects as an authoritative interpretation, in particular, ‘in providing an agreed understanding on certain aspects of the TRIPS Agreement in paragraph 5’ and in creating a ‘binding precedent for future panels and Appellate Body reports’.17
However, even if we consider the Doha Declaration as equivalent to a non-binding authoritative interpretation, the legal effects of authoritative interpretation do not amount to ‘subsequent agreements’ or ‘subsequent practice’ within the meaning of Articles 31.3(a) and (b) of the VCLT, according to Ehlermann and Ehring. These authors state that:
From a formal point of view, it is questionable whether a panel and the Appellate Body could rely on a non-binding authoritative interpretation for more than a mere reference or confirmation of legal findings the Panels have developed independently of such authoritative interpretation.18
D Confirmation of TRIPS or New Rules for Interpreting TRIPS?
According to Correa,19 ‘spelling out some of the available flexibility [of the TRIPS Agreement] was the main objective of the Declaration on the TRIPS Agreement and Public Health’. However, there is no definition of TRIPS flexibility and there seem to have been widely diverging understandings of this concept when the Doha Declaration was adopted as it is today. Among the proposals submitted to the TRIPS Council prior to the adoption of the Doha Declaration, the EU, for the special discussion on IP and access to medicines before the Doha Ministerial, referred to flexibility as follows:
The EC and their Member States consider that procedural safeguards are important to guarantee legal security. Article 31 nevertheless leaves some flexibility in cases of national emergency and other circumstances of extreme urgency, or when the subject matter of the patent is required for public non-commercial use. Although Article 31 does not itself contain tailor-made solutions to any specific problem raised in the debate on access to health, it does leave WTO Members the freedom to determine the grounds for granting compulsory licences, provided the terms of the Article, and of other provisions of the Agreement, are met, and it allows for swift action in case of emergency or extreme urgency.20
The paper submitted on 29 June 2001 by the African Group, Barbados, Bolivia, Brazil, Dominican Republic, Ecuador, Honduras, India, Indonesia, Jamaica, Pakistan, Paraguay, Philippines, Peru, Sri Lanka, Thailand and Venezuela21 argued that the TRIPS Agreement offers flexibilities first, by its interpretation in light of the objectives and principles set forth in Articles 7 and 8, secondly, by not limiting the grounds for governments to issue compulsory licences, and thirdly, by legitimising parallel imports. The paper also indicated that such remedies for access to medicines as differential pricing should not limit the flexibility of the TRIPS Agreement in any of its provisions such as parallel trade and compulsory licensing.22
The developing country paper for Doha emphasised that Article 7 is ‘a key provision that defines the objectives of the TRIPS Agreement’23 and elaborated particularly on the notion of Article 8 as expressing the principles of the TRIPS Agreement:
In Article 8, the TRIPS Agreement affirms that Members may adopt measures to protect public health, among other overarching public policy objectives, such as nutrition and socio-economic and technological development. Any interpretation of the provisions of the Agreement should take into account the principles set forth in Article 8. The reading of such provision should confirm that nothing in the TRIPS Agreement will prevent Members from adopting measures to protect public health, as well as from pursuing the overarching policies defined in Article 8.24
Significantly, the paper ignored the phrase in fine of Article 8.1,25 which says ‘provided that such measures are consistent with the provisions of this Agreement’ (see chapters 4 and 5).
In elaborating on paragraph 5a) of the Doha Declaration, the EU seems to have mitigated this interpretation which limits the ‘object and purpose’ of the TRIPS Agreement solely to Articles 7 and 8. Thus the phrase ‘in applying the customary rules of interpretation of public international law’ was inserted, and the specific reference to Articles 7 and 8 was deleted from the final version of the Declaration, to be replaced by ‘its objectives and principles’, which are not limited to Articles 7 and 8.26 Paragraph 5a), therefore, does not seem to propose anything radically different from the method of interpretation based on the customary rules of interpretation taken by the Panel in Canada–Pharmaceutical Patents27. This Panel interpreted the objectives and principles of the TRIPS Agreement from various relevant TRIPS provisions, such as Articles 1, 27 and 28, together with the Preamble, as offering a context for interpretative purposes, rather than only Articles 7 and 8. From a legal point of view, therefore, paragraph 5a) does not seem to provide a special interpretative rule for pharmaceutical products28 within the TRIPS Agreement.
Does the Doha Declaration create a special regime for pharmaceutical products, relating to compulsory licensing?29 Paragraph 5b) says that ‘each Member has . . . the freedom to determine the grounds upon which such licences are granted’. In any case, Article 31 does not take the ‘grounds’ approach, but a ‘conditions’ approach (see chapter 4) and, therefore, the TRIPS Agreement allows Members to determine the grounds for compulsory licensing. Such grounds may be limited by the conditions delineated in Article 31 and other provisions of the TRIPS Agreement.
Members may differ in their understanding of what constitutes ‘grounds’ and what are ‘conditions’. The EC, in one of its Trade Barriers Regulation (TBR) Reports in 200830 asserted that the stipulation of ‘reasonable commercial terms’ in Article 76 of the Taiwan Patent Act31 is no more than a procedural requirement, and treating it as a substantive ground for issuing compulsory licences (ie, on the grounds of ‘high prices’) runs counter to Article 31, read in conjunction with Article 28. Likewise, a mere ‘refusal to deal’ cannot be a sufficiently substantive basis for the grant of a compulsory licence, if there are no associated competition law violations. According to the EC, if a mere refusal to deal without anti-competitive behaviour, could be grounds for compulsory licensing, the exclusive rights granted to right holders pursuant to Article 28 of TRIPS would be voided of a large part of their substantive value, since their exclusive nature could be brought to an end simply by a failure to deal, thus rendering Article 31(k) redundant. Furthermore, national competition law jurisprudence shows that the ‘refusal to deal’ of a patent owner is a permissible activity that might only be considered illegal when the patent owner is in a position of market power and the refusal is linked to abuse of that market power.32
Whatever the EC interpretations concerning specific provisions of Articles 31 and 28, the Doha Declaration ‘reiterates’ the commitment of WTO Members under the TRIPS Agreement (paragraph 4, Doha Declaration). The text of the TRIPS Agreement is not changed by the Doha Declaration (except for the above-mentioned decisions adopted by the TRIPS Council) but there are radically divergent opinions as to whether the Declaration has changed the rules of TRIPS interpretation, as we have observed. Reading and interpreting the Doha Declaration independently of TRIPS provisions or without applying the international customary rules of treaty interpretation would amount to adding a layer of ‘super-flexibility’ to the flexibilities already included in the TRIPS provisions.
Treaty provisions granting a margin of discretion to the parties may be characterised as having ‘flexibilities’. Undefined concepts of ‘flexibilities’ without any rules for interpreting their scope could offer an additional margin of discretion by showing that the TRIPS Agreement can legitimately be interpreted and implemented by national laws to alleviate TRIPS obligations without violating the Agreement. This is supported by the commonly held notion that this would ipso facto solve socio-economic problems allegedly created by IPRs.
On the question of compulsory licensing, the Doha Declaration, examined in conjunction with TRIPS Agreement provisions, does not seem to go beyond the textual flexibilities provided in the TRIPS Agreement, ie, a certain scope of national discretion; rather, it supports those provisions. Under the TRIPS Agreement, Members are free to choose the grounds on which to issue compulsory licences, provided that they followed the procedural requirements (chapters 4 and 5). The Declaration supported the legitimacy of compulsory licensing, provided that the provisions of the TRIPS Agreement are respected. The radical effects of the Declaration seem to be mainly political and moral. Developing country Members had been uncertain about the reactions of others to compulsory licences. Today, it would be more constraining for Members to bring dispute cases to the WTO regarding the ways these licences are issued relating to pharmaceuticals in light of the relevant TRIPS provisions.
On the other hand, the wording of the Doha Declaration concerning exhaustion differs slightly from the TRIPS provision on the same subject. Paragraph 5 d) of the Declaration states that:
The effect of the provisions in the TRIPS Agreement that are relevant to the exhaustion of intellectual property rights is to leave each member free to establish its own regime for such exhaustion without challenge, subject to the MFN and national treatment provisions of Articles 3 and 4.
Article 6 of the TRIPS Agreement, by contrast, provides that:
For the purposes of dispute settlement under this Agreement, subject to the provisions of Articles 3 and 4 nothing in this Agreement shall be used to address the issue of the exhaustion of intellectual property rights.
According to the latter provision, a Member is not ‘free to establish its own regime for such exhaustion without challenge’ and the exhaustion policy of a Member could be inconsistent with Article 28 TRIPS. Although this could not be dealt with by the WTO dispute settlement procedures, questions concerning the exhaustion policy could be brought to bilateral dispute procedures.
So, how would the WTO Panel interpret the Doha Declaration when dealing with TRIPS pharmaceutical cases?
Footnote 1 of Article IX of the Agreement Establishing the WTO states that ‘The body concerned shall be deemed to have decided by consensus on a matter submitted for its consideration, if no Member, present at the meeting when the decision is taken, formally objects to the proposed decision.’ The Doha Declaration could therefore be considered to have been adopted at the Ministerial Conference by consensus. The dispute organs, in practice, would have to take this into account one way or another, if the parties to a dispute invoke the Declaration. Parties that do not recognise the relevance of the Declaration to the case in dispute would have difficulties in the Declaration not being interpreted by the Panel, although the disputing party could refute the public health nature of the case, in order to place the case outside the scope of the Declaration.
The Declaration would guide the dispute settlement organs’ interpretation of relevant TRIPS provisions, and the socio-economic context of the case in question may also be looked into. This will probably occur after an analysis of relevant TRIPS provisions in accordance with the rules of customary law. The Panels and Appellate Body have interpreted flexibilities in the text of the TRIPS provisions which prima facie would permit a scope of national discretion, which may not coincide with the readings of the TRIPS provisions based on the VCLT rules of treaty interpretation.
The above exploration leads us to the following conclusion. Rather than determining its formalistic status and insisting on its effects on the TRIPS Agreement with a view to establishing a doctrine or a theory of reduced TRIPS obligations (or increased national discretion), simply accepting the moral appeal of the Doha Declaration may offer more possibilities for realistic cooperation to promote access to medicines. As we will see below, public health policies involve all kinds of private interests and industrial policies. It is therefore better to consider this Declaration not as a legally prescriptive requirement but as a moral standard and a political commitment, so access to medicines can be achieved widely and effectively in different situations.
E Implementing Paragraph 6 of the Doha Declaration
i Rising expectations
After the adoption of the Doha Declaration, training seminars and consultations on its implementation followed. Non-governmental organisations (NGOs) and some developing country diplomats discussed how to implement the Declaration and how to practice compulsory licensing. For example, in 2002, at an NGO Conference on the implementation of the Doha Declaration, B Pécoul, Director of the MSF Access to Essential Medicines Campaign at that time, stated that:
compulsory licensing has undergone a dramatic transformation: no longer a pariah, compulsory licensing is now considered an important policy tool in top-level policy circles. No developing country has actually yet issued a compulsory license on a pharmaceutical, but the way is open for compulsory licensing to become the rule rather than the exception.33
In the years prior to and after the adoption of the Doha Declaration, the WHO organised training seminars on TRIPS flexibilities that provided information on national legislation or on ARV prices. In these seminars on compulsory licensing, Article 31(f) of the TRIPS Agreement was increasingly viewed as an obstacle specifically impeding ‘developing countries that have domestic drug production capacity (e.g. India) from exporting medicines to those that do not (e.g. Togo)’.34 Article 31(f) says that compulsory licences are ‘authorized predominantly for the supply of the domestic market of the Member authorizing such use’. If Article 31(f) were to prevent the export of medicines to countries without manufacturing capacity, it was argued that ‘compulsory licensing [would be] a meaningless measure for many LDCs’.35
ii 30 August solution to paragraph 6 question
If patents in a country prohibit third party production, then compulsory licensing would, theoretically, allow either local producers to manufacture copies, or the country issuing such licences to import the medicines in question. As mentioned above, Article 31(f) of the TRIPS Agreement has been criticised for preventing a small country without production capacity from obtaining cheap medicines from abroad under a compulsory licence. Paragraph 6 of the Doha Declaration instructed the TRIPS Council to find an expeditious solution by December 2002 to the problem faced by WTO Member countries without pharmaceutical manufacturing capacity where compulsory licensing would not be effective.
Diverse proposals were made regarding a solution to this problem. Several developing countries (Bolivia, Brazil, Cuba, China, Dominican Republic, Ecuador, India, Indonesia, Pakistan, Peru, Sri Lanka, Thailand and Venezuela) proposed that the TRIPS Council should recommend that an authoritative interpretation of Article 30 (limited exceptions to the rights conferred) of the TRIPS Agreement be adopted by the General Council, so as to recognise the right of WTO Members to ‘authorise local producers to make, sell and export patented public health-related products, without the consent of the patent holder, to address public health needs in another country’.39 Because the act of ‘exporting’ is not enumerated among the exclusive rights conferred by the patent in Article 28 of the TRIPS Agreement, the paper explained, this solution does not ‘unreasonably prejudice the legitimate interests of the patent owner’, which is a requirement of Article 30. For these exporting countries, this solution had ‘the advantage of avoiding burdensome procedures relating to the grant of compulsory licences’.40
The March 2002 TRIPS Council allowed Members to present their preliminary views on the possible solution to the problem identified under paragraph 6 of the Declaration on the TRIPS Agreement and Public Health. At this meeting, four basic options were put on the table: an authoritative interpretation based on Article 30; an amendment to Article 31 in order to overcome the restriction, under Article 31(f), to the possibility to export products manufactured and/or sold under a compulsory licence; a dispute settlement moratorium with regard to the non-respect of the restriction under Article 31(f); or a waiver with regard to Article 31(f).
The EC speculated on the solutions based on Article 30 and Article 31,41 but later, in June 2002, proposed a solution based on the amendment of Article 31(f) of the TRIPS Agreement.42 The US proposed a moratorium or waiver to be approved in advance, ‘which would provide the manufacturing country with certainty that its production and export of the product under the waiver will not be subject to challenge.’43 Both the EU and the US specified that the product scope should be based on the Doha Declaration, which covers ‘the grave public health problems afflicting Africa and other developing and least developed countries, especially those resulting from HIV/AIDS, malaria, tuberculosis, and other epidemics.’ The US insisted on limiting the scope of the products to include medicines to treat ‘epidemics’, as expressed in the Doha Declaration.44 Since 2002, Members have been unable to agree on the scope of diseases to be covered by the mechanism to be adopted in response to the paragraph 6 question. Several developing countries insisted that ‘diseases’ should include chronic diseases such as asthma, obesity and diabetes, which they considered to be diseases that affect public health in developing countries. In the meantime, on 27 December 2002, the US, Switzerland and Canada decided not to resort to the WTO dispute settlement procedures, if Article 31(f) were waived by a Member.
Eligible Members are either LDCs or Members that have established insufficient or no manufacturing capacities in the pharmaceutical sector for the product in question in one of the ways set out in the Annex to Decision L/540. The products exported under the paragraph 6 system must fulfil several conditions. They are to be: (i) only the amount notified to the Council for TRIPS as necessary to meet the needs of the eligible importing Member(s); (ii) identified through specific labelling or marking and packaging and/or special colouring/shaping; (iii) provided in the quantities and with the characteristics posted by the licensee on a website (paragraph 2). For the exporting Member, adequate remuneration is to be paid to the right holder, taking into account the economic value of the authorisation for the importing Member in accordance with Article 31(h), but for the importing Member, this obligation is waived. The scope of the diseases to be covered by the paragraph 6 system was much discussed during the negotiations but ultimately remained unspecified (paragraph 3).
The importing Members must take reasonable measures within their means against the risk of trade diversion, to prevent re-exportation of the products imported into their territories under the system, with technical or financial assistance from developed country Members if the importing Member is a developing or LDC country (paragraph 4). Members must ensure the availability of effective legal means to prevent the diversion of the products produced under the paragraph 6 system (paragraph 5). ‘With a view to harnessing economies of scale for the purposes of enhancing purchasing power for, and facilitating the local production of, pharmaceutical products’, the regional trade agreement (RTA) falls under the paragraph 6 system if at least half of the RTA Members are LDCs and the Members concerned share the health problem in question. In this case, the obligation under Article 31(f) of a Member of the RTA is waived (paragraph 6(i)). This will not prejudice the territorial nature of the patent rights in question, but regional patents to be applicable in the above Members should be promoted, and developed countries should provide technical cooperation to this end (paragraph 6(ii)). Members recognise the desirability of promoting the transfer of technology in order to overcome the problem identified in paragraph 6 of the Declaration (paragraph 7). The Council for TRIPS shall review annually the functioning of the system (paragraph 8). This Decision is without prejudice to the rights, obligations and flexibilities that Members have under the provisions of the TRIPS Agreement other than paragraphs (f) and (h) of Article 31 and also to the extent to which pharmaceutical products produced under a compulsory licence can be exported under the present provisions of Article 31(f) of the TRIPS Agreement (paragraph 9). Non-violation complaints are not applied concerning measures taken in conformity with the waivers contained in this Decision (paragraph 10). Decision L/540 terminates for each Member on the date on which an amendment to the TRIPS Agreement replacing its provisions takes effect for that Member (paragraph 11; see below).
When Decision L/540 was adopted on 30 August 2003, the Chairperson stated that: ‘before adopting this decision, I would like to place on the record this Statement which represents several key shared understandings of Members regarding the decision to be taken and the way in which it will be interpreted and implemented.’46 His first point was that the system to be established based on paragraph 6 of the Doha Declaration should be used in good faith to protect public health and ‘not be an instrument to pursue industrial or commercial policy objectives.’ His second point was that anti-diversion measures (special colouring or shaping) should be applied ‘not only to formulated pharmaceuticals produced and supplied under the system but also to active ingredients produced and supplied under the system and to finished products produced using such active ingredients’. His third point was that ‘any issues arising from the use and implementation of the Decision must be resolved expeditiously and amicably’.
F The First Amendment to the TRIPS Agreement
Paragraph 11 of Decision L/540 instructed47 the TRIPS Council to initiate, by the end of 2003, work on the preparation of an amendment to the TRIPS Agreement with a view to its adoption within six months.48
On 6 December 2005, after a lengthy debate on which method to use to transform the 30 August 2003 waiver into a formal amendment, the General Council49 adopted Decision L/641 amending Article 31 of the TRIPS Agreement.50 Decision L/641 adopts a Protocol of Amendment which, upon the entry into force of the Protocol pursuant to Article X of the WTO Agreement, should be amended as set out in the Annex to this Protocol, ‘by inserting Article 31 bis after Article 31 and by inserting the Annex to the TRIPS Agreement after Article 73’. The new Article 31 bis and Annex of the TRIPS Agreement are attached to the Protocol of Amendment, which, in turn, is attached to Decision L/64151. Article 31 bis allows pharmaceutical products manufactured under compulsory licences to be exported to countries lacking production capacity. An annex to the TRIPS Agreement sets out definitions and notification procedures for avoiding the diversion of pharmaceuticals to other markets, developing regional systems to allow economies of scale, and annual reviews in the TRIPS Council. An ‘appendix’ to the annex (originally an annex to the 2003 decision) deals with assessing lack of manufacturing capability in the importing country.
One of the reasons why it took more than two years to adopt the text for the amendment was uncertainty over whether the Chairperson’s statement of 30 August 2003 would be part of the amendment, notably the statement that the system drawn up relating to the problem posed by paragraph 6 of the Doha Declaration ‘would not be used as an instrument to pursue industrial or commercial policy objectives’. The announcement of the 11 Members that they would only use the system as importers in situations of national emergency or other circumstances of extreme urgency was included in the Chairperson’s statement, but not in Decision L/540. The US, Canada, Japan and the Republic of Korea considered the Chairperson’s statement to fall under Article 31.2(a) VCLT and constitute an ‘agreement relating to the treaty which was made between all the parties in connection with the conclusion of the treaty’. However, there was no evidence that all parties to the TRIPS Agreement had agreed to the statement. The US government insisted that the Chairperson’s statement, together with Decision L/540, form part of the August 30 agreement and that this statement be included as a footnote to the amended Article 31(f). It was decided that Decision L/540 would be reproduced in the amendment and that the Chairperson’s statement would be read when the amendment to Article 31 was adopted.
The entry into force of this amendment requires the ratification by two-thirds of the WTO Members. Upon ratification, the amendment becomes immediately effective in the countries that ratify it. The deadline was originally set for 1 December 2007 but the General Council extended it to 31 December 200952 and then to 31 December 2011.53 For each of the remaining Members, the waiver will continue to apply until they accept the amendment and it takes effect.
II TEN YEARS AFTER THE DOHA DECLARATION
A Compulsory Licences in Developing Countries
For a few years after the adoption in August 2003 by the TRIPS Council of Decision L/540 concerning the paragraph 6 system, certain developing countries, international organisations such as the WHO and civil society groups may have continued to expect that compulsory licensing would effectively and invariably solve the access to medicines problems. Manuals were prepared in cooperation with the World Bank.54
Compulsory licensing could have contributed to solving urgent problems when surrounding circumstances have supported it, but this required much more time than expected. Few data are available about what happened after the compulsory licences were issued. As in the case of Brazil, compulsory licensing may have primarily been a negotiating instrument for reducing the price of HIV/AIDS antiretrovirals (ARVs) in many countries.
CPTech (see chapter 8) provides web information on ‘Examples of Health-Related Compulsory Licenses’. These examples include, in particular, compulsory licences that were issued in Africa, Asia and Latin America after the Doha Declaration, mostly for ARVs or Tamiflu (oseltamivir).55
Several African countries have issued compulsory licences for ARVs since 2002, notably for standard combination drugs (3TC-d4T-NVP). In Zimbabwe, in May 2002, the Minister of Justice Affairs declared an emergency and granted a licence to Varichem Pharmaceuticals to make, use or import generic HIV/AIDS medicines for government use, for a period of six months, which was later extended.56 Mozambique,57 on 5 April 2004, and Zambia, on 21 September 2004, granted the local subsidiaries of Pharco (Egypt) licences to produce a fixed-dose combination (3TC-d4T-NVP) at royalties to the originator not exceeding 2 per cent and maximum 2.5 per cent of sales, respectively.58
The Zambian order of licence explains that the three originators had refused a licence for the combination drug to be marketed in Zambia.59 The situation surrounding the issuance of a compulsory licence is unclear, and the country appears to rely on the importation of Indian generics. CPTech provides information on compulsory licences for similar purposes in Cameroon in January 2005,60 Ghana in October 2005 and Eritrea in June 2005,61 but no further information is available on what happened afterwards.
On 29 September 2004, the Minister of Trade and Consumer Affairs in Malaysia ordered a licence to import didanosine (ddI), zidovudine (AZT) and lamivudine+zidovidine (Combivir) from India (Cipla) as supplies for public hospitals for two years. However, an agreement was then reached between the Government and the originator.62 The royalty rate to the originator is 0.5 per cent of the net selling value.63
On 25 November 2005, Taiwan granted compulsory licensing for Tamiflu (oseltamivir) antiviral for the treatment of avian flu,64 with a view to developing a stockpile for the national inventory through 31 December 2007. Actual manufacturing by Taiwanese companies did not seem to occur and cooperation was re-established with the right holder.
On 29 November 2006, the Thai Government announced a compulsory licence to import (from India) and locally produce efavirenz for government use until 31 December 2011, and that a royalty fee of 0.5 per cent of the Government Pharmaceutical Organization (GPO) total sale value of the imported or locally produced efavirenz would be paid to the right holder.65 The GPO announced that they would start producing locally in June 2007 at a price of less than half the originator price.66This, however, has not yet been achieved. According to the GPO, domestic production of efavirenz will start in 2011.67 On 25 January 2007, the Government announced two additional compulsory licences for government use of antiretroviral Kaletra (LPV+RTV) and the heart disease drug Plavix (clopidogrel bisulphate).68 The royalties to the originator were fixed at the same rate as efavirenz.
On 4 May 2007, the Brazilian Government issued a decree allowing for the production or importation of efavirenz without the consent of the right holder for use in the national HIV/AIDS programme at a royalty rate of 1.5 per cent.74 The decree followed negotiations to obtain a 40 per cent price reduction, which seems not to have been achieved. In Brazil, compulsory licensing has primarily been a negotiating tool for price agreements. The prices of ARVs in Brazil are generally lower than in some small and poor countries where the prices of patented drugs are extremely high. The following data by UNITAID75 shows that the price of Kaletra in Brazil is even lower than that procured by the Global Fund (see chapter 8).
Prices of Kaletra: differences by country (2005)
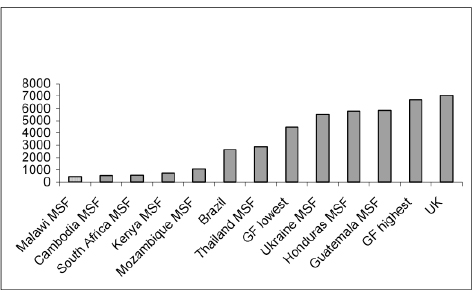
‘Intellectual Property Rights and Medicines Procurement Patent Pools’, Note by Médecins Sans Frontières (MSF) for consideration by the Ministry of Foreign Affairs (France) and UNITAID, 1 June 2006.
GF=Global Fund
On 14 April 2010, Ecuador granted a compulsory licence for ritonavir, an antiretroviral drug, to Eskegroup SA, the local distributor for Cipla.76 According to IP Watch, the method of calculating royalties that was adopted was the ‘tiered royalty method’ (TRM), recommended in the UNDP/WHO document, ‘Remuneration guidelines for non-voluntary use of a patent on medical technologies’.77 The TRM takes as a base royalty 4 per cent of the high-income country price, adjusted to relative income per capita, and for countries facing a particularly high burden of disease, relative income per person with the disease. According to this document, the TRM ‘provides a more rational framework for sharing the costs of research and independent of manufacturing costs, and vary directly with proxies for therapeutic value (the high income price) and capacity to pay.78
B Under WTO’s Export Licensing System
i Options under the paragraph 6 system
All WTO Member countries are eligible to use the paragraph 6 system adopted on 30 August 2003. When Decision L/540 was made by the WTO General Council, 23 developed countries79